.
IntroductionAndreas Gruentzig pioneered coronary angioplasty, but we are probably indebted to him for far more than this. He instigated a more creative approach to percutaneous intervention, thus inspiring other “revolutionaries” such as Amplatz, Lock, Bonhoeffer and Cribier, who extended the field of cardiac intervention to territories other than that of the coronary circulation. Structural heart disease interventions represent a new branch of percutaneous treatments covering a wide range of congenital and acquired diseases that were previously treated surgically or simply not addressed. The term structural heart disease has been widely used to refer to a group of noncoronary heart diseases ranging from septal defects to acquired valvular disease. This new branch of percutaneous intervention has several distinguishing characteristics: a) most of the disorders require a multidisciplinary approach that includes cardiac imaging specialists, clinical cardiologists, interventional cardiologists, interventional pediatricians, and cardiac surgeons among others; b) a thorough study is essential to select the patients who will benefit from percutaneous intervention; c) it requires continuous training and knowledge of the different materials and devices needed to treat the different diseases, and d) the cardiac catheterization laboratory should be adapted to perform hybrid procedures.
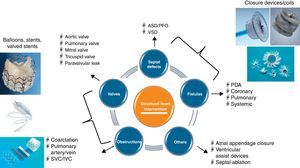
The following structural conditions can be grouped together: a) septal defects; b) valvular heart disease; c) vascular obstructions; d) fistulas, and e) other conditions. Roughly speaking, the approach can be simplified into 2 groups of interventions: a) those involving the removal of an obstruction in a vascular channel or valve using dilatation procedures with balloons, stents, or valved stents, and b) those involving the occlusion of an abnormal communication between 2 cardiac chambers or vascular channels treated with closure devices or coils (Figure 1).
Figure 1. Diagram of the different types of interventional procedures in structural heart disease. ASD, atrial septal defect; IVC, inferior vena cava; PDA, patent ductus arteriosus; PFO, patent foramen ovale; SVC, superior vena cava; VSD, ventricular septal defects.
Although structural heart disease interventions are well-established due to transcatheter aortic valve implantation, in this review we leave aside valvular surgery because it has already been addressed in the Revista Española de Cardiología and is better known.1, 2 Thus, this article briefly describes several conditions for which percutaneous intervention has become a first-line treatment option.
Septal defect Patent Foramen OvaleSince 1877, when Cohnheim described paradoxical embolism whose origin was a patent foramen ovale (PFO),3 the percutaneous closure of this abnormality has remained a matter of debate. It has been estimated that PFO occurs in about one quarter of the general population, and PFO has been recognized as a mediator of various diseases such as paradoxical embolism, orthostatic desaturation observed in orthodeoxia-platypnea syndrome, decompression sickness observed in divers, and migraine, among others.
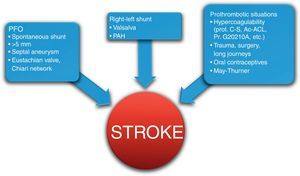
Paradoxical embolism occurs when a thrombus of venous origin reaches the arterial circulation through the PFO. The thrombus is rarely observed through the PFO, and thus paradoxical embolism is a presumptive diagnosis and should be considered in the following cases: a) an arterial source of the thromboembolism is not observed; b) potential right-left shunting through the PFO on echocardiography is observed, and c) thrombotic material in the venous system is observed. However, the clinical guidelines in neurologyand cardiology do not offer a consensus opinion and controversial studies such as the CLOSURE I trial have failed to clarify the issue of the benefits of percutaneous closure vs antiplatelet therapy.4 Part of this controversy may be due to the heterogeneity of factors that are involved in and influence cryptogenic stroke: the patient's clinical characteristics (age<55 years has a relative risk [RR]=3), prothrombotic states, venous return problems such as May-Thurner syndrome that increases the risk of venous thrombosis, and PFO characteristics that increase the possibility of right-left shunting (Figure 2, Figure 3). Therefore, patients who have accumulated multiple factors that increase the risk of recurrence, who experience a recurrence while receiving medical treatment, or who cannot tolerate medical treatment are candidates for percutaneous closure. In addition, we must take into account the low rate of procedural complications (<1%) and the reduced number of events observed after the closure procedure described in several meta-analyses (from 4.9% to 2.9%).5
Figure 2. Factors involved in the etiology of paradoxical stroke through the patent foramen ovale. Ac-ACL, anticardiolipin antibodies; PAH, pulmonary arterial hypertension; PFO, patent foramen ovale; Pr. G20210A, prothrombin G20210A mutation; prot. C-S, protein C and S deficiency.
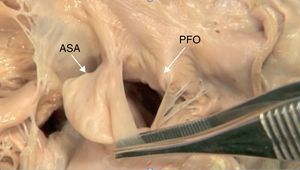
Figure 3. Anatomy of a patent foramen ovale with a giant atrial septal aneurysm. ASA, atrial septal aneurysm; PFO, patent foramen ovale.
Orthodeoxia-platypnea syndrome is characterized by desaturation on standing due to right-left shunting through the PFO, corrected by changing to a supine position. It is a clear indication for closure; in contrast, closure appears to be more than questionable in cases of migraine and decompression sickness.
Atrial Septal DefectAtrial septal defect (ASD) is a direct communication between the atrial chambers permitting left-right shunting. It represents 22% to 30% of congenital heart disease in adults and is more prevalent in women (2:1). Many of these abnormalities go unnoticed during childhood; when the shunt is significant, however, exertional dyspnea is observed in 30% during the third decade of life and 75% during the fifth.6 There are different types of ASD (ostium secundum, ostium primum, sinus venosus, and coronary sinus). The most common (70%) is ostium secundum ASD and in theory this is the only type that can be approached percutaneously; paradoxically, this is due to an abnormality of the ostium primum.
Percutaneous closure of the ASD is indicated in patients with dilatated right chambers and good margins around the defect (Table 1).7 Before closure, a full diagnostic study using transesophageal echocardiography (TEE)8 is needed to determine the size of the ASD, the presence of margins, and the absence of other associated defects. The 3-dimensional (3D) mode is useful to determine the anteroposterior and superoinferior diameters of the defect, which helps to discern the presence of more than 1 defect in the septum (Figure 4). Magnetic resonance imaging is useful to noninvasively calculate the degree of shunting and pulmonary resistance and is essential in case of any doubts about a possible anomalous pulmonary venous return.
Table 1. Indications for Percutaneous Closure of Atrial Septal Defects Based on the American College of Cardiology/American Heart Association 2010 Clinical Practice Guidelines.
| Indications for ASD closure | |
| Class I | Right ventricular dilatation with or without associated symptoms |
| Class IIa | Paradoxical embolism or orthodeoxia-platypnea syndrome |
| Class IIb | Presence of net left-to-right shunt when the pulmonary arterial pressure is less than two-thirds the systemic pressure or pulmonary vascular resistance is less than two-thirds systemic vascular resistance and when responsive to pulmonary vasodilators or positive occlusion test of the defect |
| Criteria for percutaneous closure | ASD with a minimum diameter >5 and <40mm in echocardiographic study |
| Good edges (>5 mm) from the defect to the surrounding structures including the superior and inferior vena cava, coronary sinus, atrioventricular valves, and pulmonary veins | |
| Contraindications to percutaneous closure | All septal defects that are not of the ostium secundum type, including defects of the ostium primum, venous sinus, and coronary sinus |
| Avoid those with very aneurysmatic septum or multifenestrated defects in which there is an observed scarcity of surrounding tissue | |
| Other options should be considered in the case of allergies to nickel or contraindication to antiplatelet therapy |
ASD, atrial septal defect.
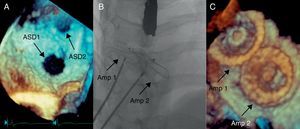
Figure 4. Closure of two atrial septal defects observed in the same patient. A, 3-dimensional transesophageal echocardiography study showing two distinct defects in the ostium primum. B, Implantation of two Amplatzer® devices. Amp 1, Amplatzer® device occluding the first defect; Amp 2, Amplatzer® device occluding the second defect; ASD1, first atrial septal defect; ASD2, second atrial septal defect.
Since the first percutaneous closure, performed by King and Mills in 1975, more than 100000 devices have been implanted worldwide. Many devices are currently on the market and most use the dual-disc system for closure. The implantation procedure can be guided by TEE, the main advantage being that the size of the defect can be accurately measured without resorting to the conventional sizing balloon. However, intracardiac echo imaging provides support similar to TEE (except for the 3D mode, currently in development), with the advantage of avoiding the need for general anesthesia, making the procedure short and tolerable; the number of supporters of intracardiac echo imaging has increased in recent years. Percutaneous closure has a success rate comparable to that of surgical closure, with a lower complication rate. Erosion, perhaps the most feared of the possible complications of percutaneous closure, is observed in less than 0.05% of cases and is mostly due to oversized devices (Figure 5).
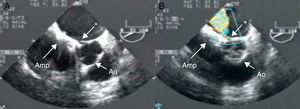
Figure 5. Aortic erosion after atrial septal defect closure with an Amplatzer® device. A, 2-dimensional transesophageal echocardiography showing a pronounced notch between the aorta and left atrium. B, Color Doppler shows a high-velocity jet between the aorta and left atrium. Amp, Amplatzer® Atrial Septal Defect Occluder device; Ao, aorta; *, erosion.
Interventricular Septal DefectVentricular septal defects (VSD) are rare in adults without congenital disease. Most VSDs in adults diagnosed by cardiologists occur in the setting of myocardial infarction and are only rarely the result of trauma or an iatrogenic artifact of cardiac surgery.9 These patients are often at high surgical risk and thus they should be considered for percutaneous closure. The mortality of postinfarction VSD is >90% with medical treatment, 46% after surgery, and 37% after percutaneous closure. Percutaneous closure has the advantage of earlier treatment, reduced mortality, and the possibility of closing inferior defects. Postinfarction VSDs present friable necrotic tissue, such that the rate of residual shunts after device closure is high (80%). However, this procedure increases patient survival and many of these residual shunts are well tolerated and can be subsequently corrected (Figure 6).
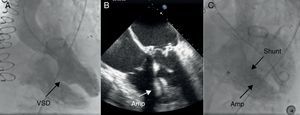
Figure 6. Postinfarction ventricular septal defect in a patient who underwent triple bypass and mitral-aortic valve replacement. A, Ventriculography in left cranial projection showing a large ventricular septal defect forming a communication between the left and right ventricles. B, Transesophageal echocardiography showing the correct placement of the Amplatzer® Atrial Septal Defect Occluder device. C, Ventriculography after implantation of the device shows a residual shunt (residual flow at the upper edge of the device). Amp, Amplatzer® Atrial Septal Defect Occluder; VSD, ventricular septal defect.
The other group of VSDs encountered by cardiologists in adults consists of muscular VSDs which, although small, are associated with a history of endocarditis. In these cases, percutaneous closure should be considered as the treatment of choice after effective treatment of the endocarditis (Table 2).7 The perimembranous closure device has not yet received Food and Drug Administration approval.
Table 2. Indications for Percutaneous Closure of Ventricular Septal Defect Based on the American College of Cardiology/American Heart Association 2010 Clinical Practice Guidelines.
| Indications for VSD closure | |
| Class I | QP/QS>2 or signs of left ventricular overload |
| Class I | History of infective endocarditis |
| Class IIa | QP/QS>1.5 and when pulmonary arterial pressure is less than two-thirds the systemic pressure or pulmonary vascular resistance is less than two-thirds systemic vascular resistance and in the presence of systolic or diastolic left ventricular dysfunction |
| Criteria for percutaneous closure | Only type IV or muscular VSD are candidates for percutaneous closure (IIb), although there is extensive experience with type II or perimembranous VSD |
| Postinfarction VSD rejected for surgery or residual shunt after surgery | |
| Good edges (>4 mm) from the defect to the surrounding structures including the aortic, pulmonary, mitral, and tricuspid valves | |
| Contraindications to percutaneous septal defects | All nonmuscular septal defects, such as type I or subpulmonary septal defects and type III or atrioventricular canal defects. Uncertainty remains regarding type II or perimembranous VSD |
| In the case of perimembranous VSD, it should be avoided in those presenting aortic valve prolapse or very aneurysmatic septum | |
| Other options should be considered in the case of nickel allergies or contraindications to antiplatelet therapy |
VSD, ventricular septal defect.
A TEE study and TEE monitoring are required before and during the procedure. Multiple projections are needed to fully assess the VSD, including the transgastric view (to outline the defect), four-chamber view, and basal short-axis view. It is important to determine whether aortic regurgitation is present. Transthoracic echocardiography may provide better resolution than TEE for defects in the most-apical region.
Percutaneous closure provides good results and a low complication rate. Atrioventricular block is one such complication, although the incidence is no higher than in patients who have undergone surgical closure.10
Aneurysms of the Coronary Sinus and Ventricular PseudoaneurysmsAneurysms of the coronary sinus and ventricular pseudoaneurysm are rare diseases. Sinus of Valsalva aneurysms are usually congenital defects; the most common are those that affect the right sinus and noncoronary sinus. They are asymptomatic until a rupture occurs that opens into the right ventricle or right atrium leading to left-right shunting. Severe aneurysm can cause atrioventricular block, aortic regurgitation, or subvalvular pulmonary stenosis only in exceptional cases. The traditional treatment has been surgical, with a mortality rate of 2%. If the valve is not very distorted and severe regurgitation is not present, percutaneous closure can be an alternative to surgery, although published series are scarce.
Ventricular pseudoaneurysms are a rare but serious complication of myocardial infarction. Treatment is surgical, but many patients are at high operative risk and in these cases percutaneous closure is a suitable alternative. In recent series of patients at high surgical risk, a success rate of 100% has been achieved by using computed tomography angiography (CTA) and 3D TEE to successfully guide the procedure.11
Fistulas Patent Ductus ArteriosusDuctus arteriosus is not a fistula as such, but a persistent communication between the aorta and pulmonary artery. The American College of Cardiology/American Heart Association guidelines indicate closure in the event of left ventricular overload with dilatation (class I), endarteritis (class I), and even slight asymptomatic ductus arteriosus (class IIa).7 When this is diagnosed in adults, percutaneous closure is the treatment of choice because calcification of the ductus increases surgical complications and the results of using percutaneous devices have been very good, with low complication rates. CTA is the best imaging technique to study the anatomy of the ductus, as its size can be measured at its narrowest point, as well as the size and shape of the ampulla (Figure 7). When the ductus is small (between <2.5mm and 3mm), closure can be performed with coils; a larger ductus can be closed with an Amplatzer® Duct Occluder device (St. Jude Medical, St. Paul, Minnesota, United States).
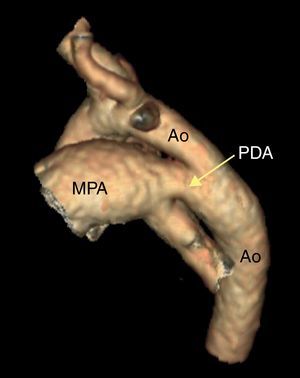
Figure 7. Computed tomography angiography with 3-dimensional reconstruction of a ductus arteriosus. Ao, aorta; MPA, main pulmonary artery; PDA, patent ductus arteriosus.
Coronary FistulasCoronary fistulas are congenital malformations in which there is direct communication between a coronary artery and a cardiac chamber, pulmonary vein, or pulmonary artery. It is the most common abnormality of the coronary system (0.22%) and is often an incidental finding.12 However, patients may have angina, myocardial infarction, or heart failure due to the steal phenomenon. In large fistulas or medium-size fistulas that lead to documented ischemia, arrhythmias, or ventricular dilatation, percutaneous closure is indicated (American College of Cardiology/American Heart Association; class I indication)7 after a study of the trajectory of the fistula and having confirmed the possibility of complete closure with angiography and CTA. Closure can be performed with coils or Amplatzer® Vascular Plug devices, with an incidence of residual shunt of <5% (Figure 8).13
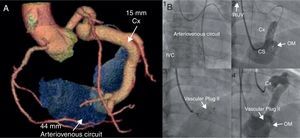
Figure 8. Coronary fistula. A, Computed tomography angiography with 3-dimensional reconstruction showing a large fistula between the distal circumflex artery and medial coronary sinus, with a narrow neck (7.1mm). B, Retrograde percutaneous closure of a coronary fistula using a 16-mm Amplatzer® Vascular Plug II, using an arteriovenous circuit (antegrade introduction of the guide in the fistula, exteriorization through the right internal jugular vein after placing the loop in the inferior vena cava). CS, coronary sinus; Cx: circumflex; IVC: inferior vena cava; OM, obtuse marginal; RIJV, right internal jugular vein. Modified with permission from Noble et al. 13
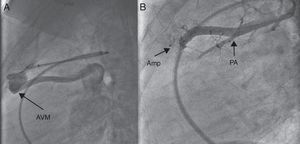
Pulmonary Arteriovenous FistulasA pulmonary arteriovenous fistula is a direct connections between the pulmonary artery and pulmonary vein leading to right-left shunting. It is commonly associated with Rendu-Osler-Weber disease (30%). These fistulas can be simple (80%) with a single feeding artery or complex (20%) with multiple arteries. If left untreated, they may lead to complications such as hemoptysis, hemothorax, transient ischemic attack, and paradoxical embolism. Both CTA and magnetic resonance angiography are appropriate techniques for the detection and description of the fistulas. Percutaneous closure is performed with coils or the Amplatzer® Vascular Plug II device, which has achieved an immediate success rate of 86% with 2% recurrence; these occur in large-diameter arteries when fewer coils are used or they are positioned in the proximal portion of the artery.14 Since there have been reports of paradoxical cerebral embolism after the procedure, dual antiplatelet therapy should be started immediately (Figure 9).
Figure 9. Closure of a pulmonary arteriovenous malformation. A, Angiogram in lateral projection showing a simple arteriovenous malformation draining into the left atrium. B, Closure with an Amplatzer® Vascular Plug II; complete occlusion of the fistula was achieved without obstructing the surrounding pulmonary branches. Amp, Amplatzer® device; AVM, arteriovenous malformation; PA, pulmonary artery. 9
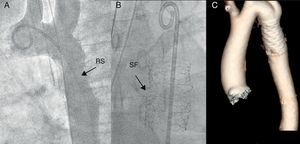
Obstructions Aortic CoarctationAortic coarctation is a narrowing of the descending aorta at the level of ligamentum arteriosum adjacent to the left subclavian artery, although this narrowing can extend to the aortic arch or isthmus. Cardiac abnormalities have to be ruled out, such as aortic bicuspid valve (80%), hypoplasia of the arch or VSD. Magnetic resonance angiography or CTA with 3D reconstruction is essential in these cases, since the presence of abundant collaterals may reduce the gradient across the obstruction and mask its severity. In addition, intracranial aneurysms (5%) can be ruled out by magnetic resonance angiography. Intervention is indicated when there is a peak-to-peak gradient>20mmHg across the coarctation or when significant narrowing is observed by radiological imaging (class I).7 Coarctation presents two challenges for adult interventional cardiologists: a) the presence of abnormal tissue in the aortic wall increases the risk of dissection or rupture in the area of coarctation, and b) many patients are referred for reoperation after stent implantation due to the stent being too small for the patient's physical development. The availability of covered stents has partly solved both problems, since the areas of rupture and dissection can be covered and the subsequent formation of pseudoaneurysms prevented; on the other hand, existing stents can be expanded until they break and a larger stent can be implanted (Figure 10). It is a safe procedure with a success rate of 98% and a low rate of complications: vascular, 2.6%; stent migration, 4.8%; and focal rupture/dissection, 1.6%. The majority of these complications can be resolved in the catheterization laboratory.15 These patients require continuous monitoring even if the coarctation is corrected.
Figure 10. Recoarctation due to the stent being too small after the patient has grown. A, The aortogram shows a notch at the point of maximum obstruction where a peak gradient of 20mmHg was measured. B, After dilating the stent with a noncompliant balloon, the stent breaks into two fragments obtaining increased aortic caliber. C, Computed tomography angiography with 3-dimensional reconstruction showing the coated stent implanted after the initial dilation with good apposition. RS, restenosis; SF, stent fracture.
Stenosis of the Pulmonary Arteries and VeinsPulmonary vein stenosis in adults is often the result of radiofrequency ablation of the pulmonary veins or secondary to surgical correction of pulmonary vein abnormalities.8 The initial results obtained with simple angioplasty and bare-metal stents showed early restenosis because of the lack of a metal skeleton during the angioplasty procedure and the small diameter of the stents. However, the development of balloons, drug-eluting stents, and in particular biodegradable stents, could resolve the problem.
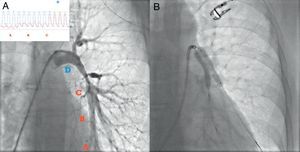
Regarding pulmonary artery stenosis, most cases are secondary to conotruncal heart defects (such as tetralogy of Fallot or pulmonary atresia) in individuals reaching adulthood. However, a smaller percentage of cases are observed in patients with vasculitis, particularly in Takayasu arteritis, following irradiation or extrinsic compression by malignant tumors. A single diseased branch may remain asymptomatic, but in most cases multiple branches are affected leading to right ventricular overload. In the case of young patients, the treatment of choice is simple or cutting balloon angioplasty; restenosis occurs in less than 12%.16 In adult patients scheduled for stent implantation, previous study with magnetic resonance angiography or CTA is essential to determine the correct stent diameter. The procedure is similar to that followed in coronary stenosis and strategies similar to those used in coronary bifurcations are sometimes needed (Figure 11).
Figure 11. Stenosis of the left main pulmonary artery in a patient with Takayasu arteritis. A, Pulmonary angiography showing significant stenosis of the left pulmonary branch and the pressure curve showing a progressive and marked increase in the gradient between points D and C. B, Two stents were implanted using the kissing technique.
Other interventions Closure of the Atrial AppendageAtrial fibrillation is the most common arrhythmia. It affects 3% to 5% of the population aged over 65 years and is the cause of 15% to 20% of ischemic strokes. The CHADS2 score is a simple, validated, and useful classification for estimating the cardioembolic risk of patients and identifying those who require anticoagulant therapy. However, patients with a higher CHADS2 score have a higher risk of bleeding (eg, patients with a CHADS2=4 have an annual risk of stroke of 11%, but a risk of bleeding of 23%).17 In addition, there is the dilemma of how to treat patients who require combined anticoagulant and antiplatelet therapy or who have experienced major bleeding and require anticoagulation therapy. Taking the above into account, and particularly considering that 90% of thrombi in patients with atrial fibrillation originate in the appendage, the exclusion of the appendage is an interesting therapeutic option. To date, the PROTECT-AF trial has been the only randomized study that has demonstrated the noninferiority of the Watchman® device (Atritech Inc., Minneapolis, Minnesota, United States) vs anticoagulation therapy with warfarin (3 events vs 4.9 events per 100 patient-years; RR=0.62, confidence interval 95%, 0.35 to 1.25).18 However, the initial complication rate impeded FDA approval of the device, although subsequent records have shown a clear reduction in adverse events, probably attributable to the learning curve (a reduction from 10% to 5%).19 Moreover, studies are underway with new devices such as the Amplatzer® Cardiac Plug and the results have shown lower complication rates than those observed in the PROTECT-AF trial. The advent of safer antithrombotic drugs, such as dabigatran and rivaroxaban, will not displace percutaneous exclusion of the atrial appendage, as there will probably be patients with major bleeding who cannot tolerate anticoagulation therapy (Figure 12).
Figure 12. Atrial appendage closure. A, The angiogram shows the Amplatzer® Cardiac Plug device partially opened and placed in the atrial appendage. B, The device has been implanted and its correct placement is confirmed by 3-dimensional transesophageal echocardiography.
Percutaneous Ventricular Assist DevicesThe development of new ventricular assist devices that are implanted percutaneously may improve treatment in patients in cardiogenic shock, since they enable a systemic flow>4 L/min and provide better support during complex coronary interventions (eg, an unprotected left main trunk with reduced left ventricular ejection fraction or aortic stenosis associated with multivessel disease). Devices such as Impella® or TandemHeart® require the insertion of a 12 to 14 Fr arterial catheter and therefore knowledge of vascular closure techniques. In addition, the TandemHeart® requires placing a cannula in the left atrium through a transseptal puncture. Thus, the implantation of these devices requires operators experienced in this type of procedure.
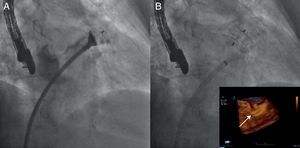
Valves Paravalvular LeaksParavalvular leaks are a relatively common complication after surgery and have been observed in up to 10% in the aortic position and 17% in the mitral position. Surgery is required in 3% of patients due to hemolysis, heart failure, or both.20 Reoperations, particularly first reoperations, have a high mortality rate and thus the percutaneous approach is becoming an attractive option among surgeons and interventionists. Percutaneous treatment leads to a reduction in the degree of regurgitation, which is less than moderate in 76% of cases; surgery-free survival at 3 years is 64% in this group of patients. Patients with a severe residual leak present event-free survival of 31%.21 Moreover, the success rate is even higher in the European and Canadian centers that have the Amplatzer® Vascular Plug III available (Figure 13).
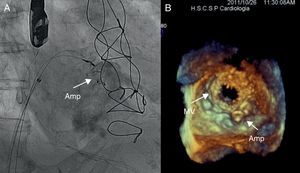
Figure 13. Closure of a severe anterolateral mitral periprosthetic leak. A, The placement of two Amplatzer® Vascular Plug III devices. B, 3-dimensional transesophageal echocardiography confirms correct placement of the devices. Amp, Amplatzer® device; MV, mitral valve.
ConclusionsInterventions in structural heart disease cover a wide range of cardiac procedures for congenital and acquired diseases including valvular diseases, septal defects, arterial or venous obstructions, and fistulas, and a variety of techniques such as closure of the atrial appendage. The positive results obtained with transcatheter valve therapy have stimulated interest among cardiologists in this field. Taken together with the development and continuous improvement of the devices, this situation augurs a promising future for this subspecialty of cardiac intervention.
Conflicts of interestNone declared.
Corresponding author: Hospital de la Santa Creu i Sant Pau, Sant Antoni Maria Claret 167, 08025 Barcelona, Spain. darzamendi@santpau.cat