Tako-tsubo syndrome produces a variable degree of transient left ventricular dysfunction. Our objective was to determine the short- and long-term prognosis of this syndrome, the incidence of and risk factors for the development of heart failure, and the influence on heart failure on the long-term outcome in our patient population.
MethodsWe prospectively recorded the clinical features and events during the hospital stay and follow-up of 100 patients with tako-tsubo syndrome. The risk factors for heart failure during hospital stay, considered as Killip class≥II, were assessed.
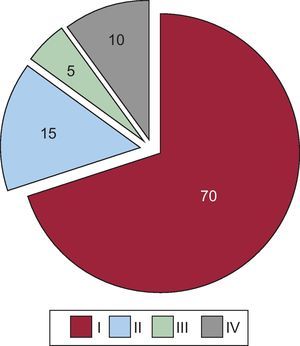
ResultsMost of the patients were women (89%), with a mean age of 68 years. The distribution according to Killip class was: Killip I, 70 patients; Killip II, 15; Killip III, 5; and Killip IV, 10. Cardiovascular risk factors, including diabetes, were common in the overall group, but were more so in the heart failure cohort. The left ventricular ejection fraction was lower in the heart failure group (51% vs 42%; P<.01). There were no differences in preadmission medications or biomarkers of necrosis. Over a median follow-up of 1380 days, the incidence of events reported during the hospital stay and long-term follow-up, both for death and the combined endpoints, was higher in the heart failure cohort.
ConclusionsAlthough the prognosis in tako-tsubo syndrome is usually good, heart failure occurs quite frequently, mainly in patients with a greater number of comorbidities and poorer previous functional class. Moreover, heart failure is associated with a higher number of early and late adverse events. The overall long-term prognosis is good.
Keywords
Tako-tsubo syndrome (TKS), also known as “broken heart syndrome”, transient apical dyskinesia, or apical ballooning, is an apparently temporary cardiomyopathy that produces a variable degree of ventricular dysfunction, predominantly in the left ventricle, and is, by definition, reversible. Occasionally related to stressful situations, in approximately half of all cases, this entity is also included in the broader group of stress cardiomyopathy.1, 2
The most striking feature of TKS is the absence of significant stenosis on coronary angiogram, although, clinically and electrocardiographically, its characteristics resemble those of acute coronary syndrome, including elevation of biomarkers of necrosis.3, 4
This syndrome was described in the early 1990s in a brief series of Japanese cases,5, 6 and was initially considered to be a rare disease; some years later, probably due to dissemination of knowledge on this entity, the existence of TKS has been confirmed with increasing frequency on every continent and in every race.7, 8 In our environment, both typical and atypical forms have been reported,9, 10 and TSK has even been diagnosed in patients with previous ischemic heart disease.11
Although benign and transient, paradoxically, TSK is not free of serious complications, mostly occurring during the hospital stay, in the acute phase; the most common adverse event is the development of variable degrees of heart failure (HF).4, 12
The purpose of this study was to determine the incidence of and risk factors for the development of HF in a case series with a final diagnosis of TKS in our environment, as well as to carry out a long-term follow-up. In addition, we analyzed the possible influence of this in-hospital complication on the disease course during the follow-up of these patients.
Methods Patient Inclusion, Study, and Follow-upIn the present study, the clinical, electrocardiographic, analytical, and characteristics were carefully and prospectively recorded, as were the events occurring during the hospital stay and follow-up of 100 consecutive patients with a diagnosis of TKS, according to the Mayo criteria1 (modified in 2008). All patients had to meet all of the following inclusion criteria and none of the exclusion criteria:
• Clinical presentation resembling that of acute coronary syndrome.
• Transient segmental left ventricular dysfunction, without significant stenosis (≥50%) or plaque rupture as the potential cause of the findings of coronary angiography performed during the hospital stay. Echocardiography and coronary angiography were carried out in all patients during the acute phase, with median delays of 0 and 1 day, respectively. Subsequent complete ventricular recovery had to be be verified by means of imaging studies (with at least a complete echocardiogram).
• New electrocardiographic abnormalities (either ST segment elevation or depression, or repolarization abnormalities expressed by deeply inverted T waves) and elevated biomarkers (especially troponin in all patients).
• Exclusion of: pheochromocytoma, intracranial involvement, previously known ischemic heart disease, severe organic valve disease, or prosthetic heart valves.
The participants were recruited from 2002 to 2010 inclusive. A post hoc (retrospective) analysis was carried out to assess the development of HF based on a dichotomous approach considering a Killip class of II or higher during the initial hospital stay to indicate the presence of HF. The degree of HF was evaluated according to the well-known Killip-Kimball classification, which establishes 4 groups: class I, no clinical or radiological signs of HF; class II, evident dyspnea, pulmonary crackles, elevated central venous pressure, and third heart sound; class III, pulmonary edema demonstrated by chest X-ray; and class IV, evidence of cardiogenic shock, according to the criteria of the SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial.13
All patients were treated according to the criteria of their attending physician, which was adjusted to the norms indicated by the clinical practice guidelines in force at each time point. The imaging study employed to verify full recovery was a complete echocardiogram. In some cases, the patient underwent additional noninvasive imaging studies (for example, cardiac magnetic resonance).
Since hospital discharge, follow-up contact has been made with all of the patients at least once a year, either in the cardiology unit or by telephone interview. During this period, the following situations were considered adverse events: all-cause readmission to the cardiology unit, recurrence of pain, and all-cause mortality, and, as the combined endpoint (a major adverse cardiac event), the development of any of these events.
Statistical AnalysisThe variables are expressed as mean (standard deviation), percentages, or median [interquartile range], depending on the quality and distribution of the variable. The tests employed to compare the variables (χ2, Student's t, ANOVA, etc.) were selected in accordance with these characteristics. The independent predictors of HF were estimated using a binary logistic regression model (multivariate analysis). The variables included in these multivariate models were selected on the basis of the results obtained in the univariate analyses, and are described in the text. The goodness of fit of the models was estimated by the Hosmer-Lemeshow test. The occurrence of events during follow-up was studied using the Kaplan-Meier technique, and the differences in course across strata, with the Breslow test. All the statistical analyses were carried out with the SPPS v15 software package (SPPS 2006, Chicago, Illinois, United States). In all analyses, a two-sided P value less than .05 was considered to indicate statistical significance.
Results Characteristics, Clinical Features, and Ancillary TestsMost of the patients included were postmenopausal women (89%), with a mean age of 68 years. The group of patients with HF during their hospital stay frequently had multiple cardiovascular risk factors (Table 1), were older and included notably higher percentages of diabetic and obese individuals. The medical histories showed that most of the patients had experienced a recent stressful event, either physical (10%) or emotional (43%). Table 2 shows the vital signs on admission and the most noteworthy baseline laboratory values, together with peak levels of biomarkers of necrosis. Upon hospital arrival, the HF group had higher heart rates and lower arterial blood pressures than did patients in Killip class I throughout their hospital stay. No significant differences were observed between the 2 groups in body temperature and the remaining analytical parameters; the mean biomarker (troponin and creatine kinase) levels were slightly higher in the HF cohort but these differences were not statistically significant.
Table 1. Baseline and Clinical Characteristics of the Overall Group and According to the Presence or Absence of Heart Failure.
| Overall (n=100) | Without HF (n=70) | With HF (n=30) | P a | |
| Women | 89 | 87.1 | 93.3 | .36 |
| Age, years b | 68.0 (13.2) | 66.0 (13.2) | 72.9 (12.0) | .01 |
| HT | 68 | 71.4 | 60.0 | .26 |
| DLP | 50 | 51.4 | 46.7 | .66 |
| DM | 18 | 12.9 | 30.0 | .04 |
| Smoker | 31 | 30.0 | 33.3 | .74 |
| Family history of IHD | 19 | 26.5 | 3.7 | .01 |
| Obesity (BMI≥30) | 24 | 19.1 | 37.9 | .04 |
| Hyperuricemia | 3 | 1.4 | 6.9 | .15 |
| OSAS | 1 | 1.5 | 0 | .51 |
| Previous NYHA functional class | <.01 | |||
| I | 64 | 77.1 | 33.3 | |
| II | 31 | 21.4 | 53.3 | |
| III | 5 | 1.5 | 13.3 | |
| Stressor | .75 | |||
| Emotional | 43 | 44.3 | 40.0 | |
| Physical | 10 | 8.6 | 13.3 | |
| None | 47 | 47.1 | 46.7 | |
| Chest pain c | <.01 | |||
| Yes | 88 | 95.7 | 70.0 | |
| No | 12 | 4.3 | 30.0 |
BMI, body mass index; DLP, dyslipidemia; DM, diabetes mellitus; HF, heart failure; HT, hypertension; IHD, ischemic heart disease; NYHA, New York Heart Association; OSAS, obstructive sleep apnea syndrome.
The data are expressed as percentages or mean (standard deviation).
a χ2 test.
b Student's t test.
c Major reason for seeking medical attention.
Table 2. Data Collected at the Time of Hospital Admission and Peak Levels of Biomarkers of Myocardial Necrosis.
| Overall (n=100) | Without HF (n=70) | With HF (n=30) | P a | |
| SBP, mmHg | 143.0±35.8 | 150.1±32.6 | 127.0±38.0 | .04 |
| HR, bpm | 86.0±21.0 | 81.4±16.5 | 96.8±27.3 | .02 |
| Body temperature, °C | 36.2±0.4 | 36.2±0.4 | 36.4±0.5 | .43 |
| Creatinine | 0.95±0.4 | 0.90±0.2 | 1.0±0.3 | .10 |
| Leukocytes | 9.8±3.3 | 9.5±2.9 | 10.6±3.9 | .15 |
| Hemoglobin | 13.5±1.4 | 13.5±13.0 | 13.5±15.0 | .89 |
| Platelets | 250.0±70.0 | 249.1±67.8 | 251.3±77.3 | .80 |
| Peak TnI b | 4.2 [2.0-8.9] | 3.9 [2.1-7.8] | 6.6 [1.8-11.9] | .27 |
| Peak CK b | 200.5 [123.5-329.8] | 196 [121-325] | 234 [140-462] | .23 |
CK, creatine kinase; HF, heart failure; HR, heart rate; SBP, systolic blood pressure; TnI, troponin I.
Unless otherwise indicated, the data are expressed as mean±standard deviation or median [interquartile range].
a Student's t test.
b Mann-Whitney U test.
Table 3 shows the results of the ancillary tests. The electrocardiogram revealed a higher but nonsignificant percentage of ST segment elevation, with a greater mean QT interval corrected for heart rate (QTc) in the HF group. In the echocardiogram, the left ventricular ejection fraction (LVEF) on admission was lower in the cohort in Killip class≥II (mean, 51% vs 42%; P<.01). Subsequently, although all the patients achieved a normal LVEF after normalization of the ventricular segmental abnormalities, the mean in the group with in-hospital HF continued to be slightly lower (60% vs 65%; P<.01). Significant valve disease (grade 2 or higher) was observed in 25% of the patients, more frequently in the group with HF (Table 3). The majority corresponded to mitral regurgitation (mild in 18 patients, moderate in 6, and severe in 1, due to systolic anterior motion), but moderate aortic regurgitation was reported in 2 patients. In 33 patients, the echocardiogram performed prior to discharge confirmed complete correction of the segmental abnormalities. In general, the median time to ventricular recovery was 74 days. The findings on catheterization (median, 1 day [0-2 days]), which revealed no angiographic evidence of significant coronary lesions or plaques as the cause of the condition in any of the patients, were congruent with the initial transthoracic echocardiogram in terms of the LVEF.
Table 3. Data of Interest From the Ancillary Tests.
| Overall (n=100) | Without HF (n=70) | With HF (n=30) | P a | |
| Electrocardiogram | ||||
| Sinus rhythm | 96 | 95.7 | 96.7 | .82 |
| ST elevation on first ECG | 58 | 59.7 | 62.1 | .82 |
| ST elevation b | 60 | 62.1 | 65.5 | .75 |
| ST depression b | 5 | 3.2 | 10.7 | .13 |
| Inverted T waves b | 87 | 86.4 | 89.3 | .69 |
| Peak QTc (V3) b | 512.8±72.0 | 499.5±67.8 | 543.3±73.6 | .01 |
| Transthoracic echocardiogram | ||||
| Initial LVEF, % c | 48.1±11.0 | 50.9±10.1 | 41.8±11.8 | <.01 |
| Valve disease (> mild) | 25 | 18.6 | 40.0 | .02 |
| LVOT pressure gradient (>25 mmHg) | 9 | 7.1 | 13.3 | .32 |
| LVEF after follow-up, % c | 63.9±7.2 | 65.3±7.3 | 60.4±5.9 | <.01 |
| Coronary angiography | ||||
| Emergency catheterization | 33 | 32.3 | 34.5 | .83 |
| Right coronary dominance | 79 | 81.5 | 75.9 | .02 |
| LVEF, catheterization, % c | 53.5±11.0 | 55.3±11.5 | 48.8±10.1 | .01 |
ECG, electrocardiogram; HF, heart failure; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; QTc, QT segment corrected for heart rate.
Date are expressed as a n (%) or mean±standard deviation.
a χ2 test.
b Throughout the entire hospital stay.
c Student's t test.
Most of the patients (n=70) showed no evidence of HF on admission but, in some (n=10), the signs and symptoms were so marked that they were indicative of cardiogenic shock (Figure 1).
Figure 1. Pie chart showing the distribution of the 100 patients according to Killip class (I to IV) during hospital stay.
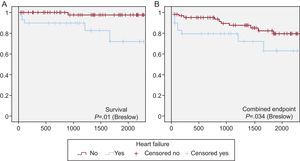
Adverse Events and Follow-upMost of the complications occurred during the hospital stay, although some occurred over the long-term follow-up period, which reached a median of 1380 days [755-1941 days] in the overall cohort. Aside from the development of HF (Table 3), the remaining complications are detailed in Table 4. The data show that the incidences of all types of complications, both during hospital stay and after discharge, were higher in the group of patients with HF. Moreover, the median duration of hospital stay was longer in the HF cohort (9.5 days vs 6 days; P<.001). When the patients were stratified according to the presence or absence of in-hospital HF, over the long term, patients with HF had a poorer disease course, whether the outcome was death or the combined endpoint (major adverse cardiac event) (Figure 2A and B).
Table 4. Adverse Events During Hospital Stay and Follow-up, %.
| Overall (n=100) | Without HF (n=70) | With HF (n=30) | P | |
| Hospital stay | ||||
| LV apical thrombus | 5 | 2.9 | 10.0 | .14 |
| Arrhythmias | 32 | 27.1 | 44.3 | .27 |
| New episode | 11 | 10.0 | 14.8 | .53 |
| Other complications (infection, vascular access, etc.) | 20 | 13.6 | 35.7 | .01 |
| Death | 0 | 0 | 0 | 1 |
| Postdischarge follow-up | ||||
| MACE | 20 | 15.7 | 30.0 | .10 |
| Readmission to cardiology unit | 16 | 14.3 | 20.0 | .47 |
| Recurrence of TKS | 4 | 1.4 | 10.3 | .04 |
| Death | 6 | 1.4 | 16.7 | .03 |
| Cardiovascular mortality | 3 | 1.4 | 6.7 | .15 |
HF, heart failure; LV, left ventricular; MACE, major adverse cardiac event (combined endpoint involving all-cause readmission to the cardiology unit, recurrence of pain, and all-cause mortality); TKS, tako-tsubo syndrome.
Figure 2. Kaplan-Meier curves showing actuarial event-free survival. A: Non-survivors. B: Onset of the combined endpoint (death or all-cause readmission to a cardiology unit). The patients were stratified according to whether they had developed heart failure (blue) or had not (red) during their hospital stay. The comparison between strata was carried out using the Breslow test.
Treatments ReceivedThe groups were compared in terms of the treatments they were receiving prior to their first hospital admission, during the hospital stay, and at the time of discharge (Table 5). There were no marked differences in the medications taken prior to admission. In those administered during the hospital stay, there were the logical differences stemming from the management of HF, including vasoactive support, balloon counterpulsation, and mechanical ventilation. Among the drugs recommended at discharge, there were higher percentages of diuretics in the HF cohort.
Table 5. List and Comparison of the Treatments Most Frequently Received by the Patients Prior to Admission, During Their Stay in the Coronary Care Unit-Intensive Care Unit, in the Ward, and at Discharge, %.
| Treatment | Overall (n=100) | Without HF (n=70) | With HF (n=30) | P a |
| Prior to admission | ||||
| ASA | 16 | 17.1 | 13.3 | .63 |
| Clopidogrel | 2 | 1.4 | 3.3 | .53 |
| Anticoagulants | 10 | 11.4 | 6.7 | .46 |
| Nitroglycerin | 2 | 1.4 | 3.3 | .55 |
| Diuretics | 16 | 14.3 | 20.0 | .47 |
| Statins | 14 | 10.0 | 23.3 | .07 |
| Calcium channel blockers | 10 | 10.0 | 10.0 | 1 |
| Beta-blockers | 11 | 10.0 | 13.3 | .62 |
| ACE inhibitors/ARB | 29 | 28.6 | 30.0 | .88 |
| Glucocorticoids | 6 | 7.1 | 3.3 | .46 |
| Anxiolytics | 12 | 11.4 | 13.3 | .78 |
| Antidepressive agents | 8 | 7.1 | 10.0 | .62 |
| Oral antidiabetic drugs | 9 | 8.6 | 10.0 | .81 |
| Insulin | 5 | 4.3 | 6.7 | .61 |
| Admission to the coronary care unit | ||||
| Inotropic agents | 11 | 1.4 b | 33.3 | <.001 |
| Balloon counterpulsation | 2 | 0 | 6.7 | .02 |
| Noninvasive mechanical ventilation | 3 | 0 | 10.0 | .007 |
| Mechanical ventilation | 6 | 1.4 | 16.7 | .003 |
| Anti-GPIIb/IIIa | 31 | 35.7 | 20.0 | .11 |
| Fibrinolysis | 3 | 4.3 | 0 | .25 |
| Treatment at discharge | ||||
| ASA | 69 | 74.3 | 56.7 | .08 |
| Clopidogrel | 8 | 10.0 | 3.3 | .26 |
| Anticoagulants | 18 | 15.7 | 23.3 | .36 |
| Nitroglycerin | 15 | 12.9 | 20.0 | .30 |
| Diuretics | 17 | 4.3 | 46.7 | <.001 |
| Statins | 55 | 55.7 | 53.3 | .82 |
| Calcium channel blockers | 30 | 27.1 | 36.7 | .34 |
| Beta-blockers | 52 | 50.0 | 56.7 | .54 |
| ACE inhibitors/ARB | 62 | 61.4 | 63.3 | .80 |
| Glucocorticoids | 9 | 8.6 | 10.0 | .81 |
| Anxiolytics | 22 | 21.4 | 23.3 | .83 |
| Antidepressive agents | 7 | 7.1 | 6.7 | .93 |
| Oral antidiabetic drugs | 8 | 8.6 | 6.7 | .74 |
| Insulin | 6 | 4.3 | 10.0 | .20 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers (angiotensin II receptor antagonists); ASA, acetylsalicylic acid; HF, heart failure.
a χ2 test.
b Dopamine-induced diuresis.
Taking into account the results of univariate analysis (Table 1, Table 2, Table 3), we performed a multivariate analysis (binary logistic regression) to assess the development of HF during hospital stay. The relevant prognostic variables (Table 6) were found to be diabetes mellitus (odds ratio [OR]=4.2), obesity, the absence of chest pain as the major reason for seeking medical attention, the presence of left-sided valve disease, hypotension, tachycardia or ventricular dysfunction, and an abnormal New York Heart Association functional class (≥II) prior to hospital admission.
Table 6. Results of Multivariate Analysis in the Overall Cohort of 100 Patients.
| OR (95%CI) | P | |
| Diabetes mellitus | 4.20 (0.77-22.96) | .09 |
| Obesity | 3.60 (0.03-15.99) | .08 |
| Chest pain as reason for seeking medical attention | 0.07 (0.01-0.45) | .005 |
| Valve disease (>mild) | 4.23 (1.02-17.49) | .04 |
| SBP>140 mmHg | 0.31 (0.08-1.20) | .09 |
| HR>100 bpm | 7.71 (1.44-41.30) | .01 |
| LVEF<45% | 4.94 (1.09-22.32) | .038 |
| Previous NYHA class (≥II) | 8.30 (2.04-33.74) | .003 |
95%CI, 95% confidence interval; HR, heart rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OR, odds ratio; SBP, systolic blood pressure.
The model included the following variables: diabetes mellitus, family history, obesity (body mass index>30), previous NYHA functional class, chest pain as the reason for seeking medical attention, age>70 years, SBP>140mmHg, HR>100 bpm, LVEF (echocardiography)<45%, and left valve disease (>mild). Calibration of the model (Hosmer-Lemeshow test) was adequate (P=.903).
TKS is occasionally included in a broader group that encompasses conditions known as acute coronary syndromes with no significant lesions on coronary angiography.14 This situation, which includes diseases of highly heterogeneous etiology, is not an uncommon problem in routine clinical practice. In fact, it can be detected in between 7% and 32% of women and between 6% and 12% of men who are admitted to hospital with suspected myocardial infarction.14 When the definitive etiology is determined, the spectrum is broad and ranges from unknown causes to myocarditis, and includes vasospasm, atherosclerosis-related myocardial infarction per se, and a considerable number of patients with a final diagnosis of TKS; this syndrome is observed in more than 10% of patients, as mentioned both in Spanish and international series.15, 16
The present report analyzes the largest series studied in a Spanish or Latin American population, with data very similar to those published by other international groups in Caucasians and other races.2, 7, 12, 17 We review the features that can be associated with the development of HF in a group of 100 patients with TKS and the influence of this complication on follow-up. The experience accumulated by several Spanish centers with cases of this type indicates that the patients who develop HF are older and have a greater number of comorbidities (diabetes mellitus, obesity, worsening of New York Heart Association functional class, etc.). The differences between the 2 groups in terms of the major symptom that led the patient to seek medical attention is clearly explained because, in the HF group, the complaint has more to do with symptoms of HF than with chest pain. Moreover, on admission, patients with HF more often present with tachycardia and normal or low arterial blood pressure. The ventricular abnormalities are obviously more marked in the HF group, which, in itself, largely explains the development of HF. Ventriculography reveals slightly higher LVEF because the echocardiographic data are usually recorded earlier and possibly already reflect the onset of a certain degree of ventricular recovery, a process that, in some patients, becomes evident within a few hours. Moreover, although not statistically significant, left ventricular outflow tract obstruction was more common in our group with HF (13.3% vs 7.1%); this, in itself, possibly together with a certain degree of valve disease (Table 3), could influence the development of symptoms.
However, although in-hospital HF is the complication most frequently reported in TKS, with an incidence of 30% in our series, it also coincides with a higher percentage of other complications, over both the short- and long-term follow-up. This situation may be a consequence of the very fact that these patients are frailer and have a greater number of comorbidities, and possibly a worse baseline status and functional class. Regardless of these considerations, such a high rate of in-hospital events is noteworthy, since TKS has classically been considered a benign disease, with no significant coronary stenosis. Thus, although the long-term prognosis appears to be good,4 prediction of in-hospital development of HF may help to allow early and more meticulous management, and perhaps to facilitate correct risk stratification of the patients at discharge, once the acute phase has passed. Therefore, the importance of HF in TKS explains the interest shown by a number of researchers, such as Madhavan et al.,18 who recently proposed a score consisting of 3 variables (age >70 years; LVEF <40%; and the presence of a physical stressor), each of which were allocated 1 point. The discriminatory power obtained was good (area under the curve=0.77; P<.001), with similar results when we applied this score to our own patients (area under the curve=0.741; P<.001). Unfortunately, this score overlooks data that are fundamental, but can be obtained easily and rapidly, such as the previous functional class, which was found to be one of the independent predictors of HF in our patients.
Although the ultimate causes of TKS remain to be clarified,1 and assuming that there is generally a negative but transient influence of circulating catecholamines19, 20, 21 in the myocardium, from the available data we can extract some specific reflections:
• During hospital stay, TKS is not free of complications, some of which are serious (an incidence of cardiogenic shock of 10%) and, thus, close follow-up and early treatment are warranted, despite the absence of significant coronary lesions.1
• The frailest patients, the elderly, and those with diabetes, tachycardia, or hypotension have the highest probability of developing HF during the course of the disease, findings that indicate a greater likelihood of subsequent readmission or death during long-term follow-up. The role of echocardiography is fundamental for the early diagnosis of ventricular dysfunction, valve disease, or possible complications (for example, intraventricular pressure gradient or ventricular thrombus), and the confirmation of their subsequent resolution.22 At the present time, we have no clinical or imaging data that enable us to conclusively predict the diagnosis of TKS and avoid the performance of coronary angiography23 during the acute phase.
• Nevertheless, certain features point to TKS: a stressor (emotional or physical, such as a fall in which the individual is unable to get up for a long time or an asthma attack), postmenopausal status, modest electrocardiographic changes, and left ventricular dysfunction with segmental abnormalities corresponding to several coronary territories.
• We have been unable to find a clear relationship between TKS and previous treatment or the development of HF during hospital stay.
• Management. There are simply no studies that establish clear recommendations for treatment. Thus, it appears to be advisable to adjust the antithrombotic therapy, prescribing anticoagulation drugs for patients with indications (atrial fibrillation, prosthesis, etc.), ventricular thrombus, or severe ventricular dysfunction. In all the patients in this report, the thrombus disappeared with acenocoumarol therapy (Sintrom) once ventricular function had been restored, leaving no sequelae. If there is marked outflow tract obstruction, vasoconstrictors should be avoided as they can exacerbate this condition. In Table 5, we provide the recommendations made to our patients at discharge. The low rate of events—resulting in a low statistical power—during follow-up hampered an adequate multivariate analysis that would allow them to be related to the complications during follow-up. Univariate analysis revealed no significant differences, except in the patients who began taking diuretics, who showed higher rates of the combined endpoint (41.2% vs 15.7%; P=.017) and death (17.6% vs 3.6%; P=.026). However, we consider it reasonable to deal strictly with the cardiovascular risk factors (diabetes mellitus, obesity) and perhaps recommend long-term antiplatelet therapy with acetylsalicylic acid. None of the patients who were prescribed acetylsalicylic acid at discharge developed adverse events attributable to this medication. Treatment with beta-blockers after discharge is optional and perhaps recommendable, given the possible etiology of the condition.
We need more detailed studies on a disease that is not that uncommon and about which there is still little epidemiological, etiological, or therapeutic information. In this respect, a Spanish TKS registry (RETAKO) is already underway, and we hope it will serve to shed some light on this condition in the coming years.
LimitationsThe observational design of the study prevents us from concluding that there are causal relationships. Nevertheless, the results obtained, which are congruent with previously published data, enable us to propose valid working hypotheses. The small number of both patients and events limited statistical power and hence the performance of a more thorough multivariate analysis during follow-up when evaluating postdischarge complications.
Ventricular recovery usually takes place within a short period of time but, in many cases, due to logistics, we are unable to detect the exact moment in which it occurs. Thus, the approximation of time is based on the date of the image in which it is definitively demonstrated.
ConclusionsIn general, although the prognosis of TKS is usually good, HF is a frequent complication (30%). This condition, more common among patients with comorbidities and worse functional classes prior to onset, is associated with a higher rate of adverse events, both during hospital stay and over long-term follow-up.
FundingProject supported by a grant from the Fundación Mutua Madrileña Automovilista (FMMA).
Conflicts of interestNone declared.
Received 12 February 2012
Accepted 14 April 2012
Corresponding author: Unidad Coronaria, Cardiología Intervencionista e Imagen Cardiovascular, Instituto Cardiovascular, Hospital Clínico San Carlos, Prof. Martín Lagos s/n, 28040 Madrid, Spain. ibnsky@yahoo.es