Transcatheter edge-to-edge repair (TEER) should be considered in patients with heart failure and secondary mitral regurgitation (MR). Angiotensin receptor-neprilysin inhibitors (ARNIs) have been demonstrated to improve prognosis in heart failure. We aimed to evaluate the impact ARNIs on patient selection and outcomes.
MethodsThe population of the Spanish TEER prospective registry (March 2012 to January 2021) was divided into 2 groups: a) TEER before the ARNI era (n=450) and b) TEER after the recommendation of ARNIs by European Guidelines (n=639), with further analysis according to intake (n=52) or not (n=587) of ARNIs.
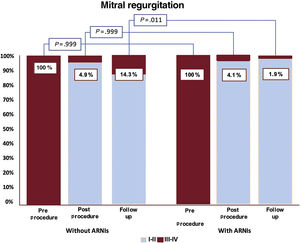
ResultsA total of 1089 consecutive patients underwent TEER for secondary MR. In the ARNI era, there was a reduction in left ventricle dilation (82mL vs 100mL, P=.025), and better function (35% vs 38%, P=.011). At 2 years of follow-up, mortality (10.6% vs 17.3%, P <.001) and heart failure readmissions (16.6% vs 27.8%, P <.001) were lower in the ARNI era, but not recurrent MR. In the ARNI era, 1- and 2-year mortality were similar irrespective of ARNI intake but patients on ARNIs had a lower risk of readmission+mortality at 2 years (OR, 0.369; 95%CI, 0.137-0.992; P=.048), better NYHA class, and lower recurrence of MR III-IV (1.9% vs 14.3%, P=.011).
ConclusionsBetter patient selection for TEER has been achieved in the last few years with a parallel improvement in outcomes. The use of ARNIs was associated with a significant reduction in overall events, better NYHA class, and lower MR recurrence.
Keywords
Secondary mitral regurgitation (MR) is the result of an imbalance between closing and tethering forces on the mitral valve secondary to annular dilatation and geometrical distortion of the subvalvular apparatus mostly due to dilated or ischemic cardiomyopathies. The first step in the management of patients with secondary MR and heart failure (HF) should be the introduction of a variety of pharmacological treatments. If symptoms persist after optimization of conventional HF therapy, including cardiac resynchronization therapy, options for mitral valve intervention should be evaluated. Transcatheter edge-to-edge repair (TEER) is a percutaneous technique that reduces MR by valve leaflet approximation in patients with HF and secondary MR.1
Recently, new pharmacotherapy has been introduced in the treatment of HF. Angiotensin receptor-neprilysin inhibitors (ARNIs) such as sacubitril/valsartan reduce the risk of HF hospitalizations and death in patients with HF and reduced ejection fraction who remain symptomatic despite angiotensin-converting enzyme inhibitors, beta-blockers, and a mineralocorticoid receptor antagonist.2–4 However, it is unknown if the extended pharmacotherapy spectrum on the treatment of patients with secondary MR and HF has an impact on the timeline selection of candidates and/or the outcomes of patients undergoing percutaneous mitral repair.
The present study aimed to evaluate the temporal trend of patients undergoing TEER and the impact of the introduction of ARNIs in routine clinical practice on patient selection and outcomes.
METHODSThis multicenter, retrospective study included data obtained from the Spanish TEER prospective registry. This registry is endorsed by the Spanish Cardiac Catheterization and Coronary Intervention working group. From March 2012 through January 2021, a total of 1089 consecutive patients at 23 centers in Spain underwent TEER for the treatment of secondary MR and were prospectively collected within a dedicated database. The decision to perform TEER was made by the local Heart Teams of each hospital. We aimed to evaluate the temporal trend and the impact of ARNIs on patient selection and outcomes. The study was approved by local ethics committees, which waived consent given the retrospective data analysis performed and the prior consent provided by the patients for the Spanish registry.
For the purpose of the present study, the population was divided into 2 groups: a) patients who underwent TEER before the ARNI era (n=450) and b) patients who underwent TEER after ARNIs had been indicated by clinical guidelines4 and were available (n=639). In this last group, patients were also divided according to the prescription of this medication (52 patients) or not (587 patients). Twelve patients were excluded due to missing information, but prescriptions (drugs and dosage) were revised for all other cases.
Procedure and deviceTEER devices have 2 arms that are opened and closed with the use of the delivery system handle. After transseptal puncture, the device is steered until it is aligned over the origin of the regurgitant jet and advanced into the left ventricle. With both arms in an open position, the device is retracted until both mitral leaflets are inside both arms at which time the arms are closed and the result is evaluated with transesophageal echocardiography. These steps can be repeated if the final result is unsatisfactory. The procedure has been described in detail elsewhere.5 Technical success was defined as the achievement of successful access, delivery, and retrieval of the device delivery system, successful deployment and correct positioning of the first intended device performed, with simultaneous freedom from emergency surgery or reintervention related to the device or access procedure.6 Currently, this therapy is recommended in clinical practice guidelines for patients with left ventricular dysfunction and significant MR that remains symptomatic despite optimal medical therapy, including the administration of ARNIs.1,7
Study endpointsThe primary endpoint was a combined endpoint of mortality and HF rehospitalizations at the 1- and 2-year follow-up following successful TEER in both the pre-ARNI and ARNI eras and, within this last group, the same endpoints according to the prescription or not of this medication.
Secondary endpoints were mortality and rehospitalization, which were also analyzed independently as secondary outcomes. Baseline differences according to the date of the procedures were also compared to determine the impact of scientific evidence on the selection of TEER candidates.
Statistical analysisCategorical variables as expressed absolute values and percentages. Continuous variables are reported as median (interquartile range [IQR]). The normal distribution of continuous variables was verified with the Kolmogorov-Smirnov test and q-q plot. Categorical variables were compared with the chi-square test and the Fisher exact test when necessary. We compared continuous variables with the Mann-Whitney U test. We analyzed time to 2-year mortality by Kaplan–Meier survival curves, which were compared using the log-rank test. Multivariate analysis through logistic regression was performed to determine independent predictors of the combined endpoint. We performed the statistical analyses with the use of R software, version 3.6.1 (R Project for Statistical Computing) and MedCalc Statistical Software version 18.9.1 (MedCalc Software bvba, Ostend, Belgium). Differences were statistically significant when at P <.05.
RESULTSStudy populationA total of 1089 patients were included in this study. The median age was 75 [68-81] years, 30.4% were women, the EuroSCORE II risk was 7.4% [5.3-12.1], and the Society of Thoracic Surgeons risk calculation was 5.6% [4.2-7.9]. Most patients were in New York Heart Association (NYHA) III-IV at the time of the intervention (87.2%). Of them, 639 patients (58%) were included after January 1, 2017 when ARNIs became available in our setting,4,5 designated the “ARNI era”. Following that date, this medication was being used in 52 patients (8.1% of the total number of patients in this period) when the TEER procedure was scheduled. Clinical and echocardiographic baseline characteristics of the global study population and according to the timeline (before or during the ARNI era) are summarized in table 1 and table 2, respectively.
Baseline characteristics of the overall study population according to pre- or post-ARNI era
| Overall study population | Pre-ARNI (n=450) | Post-ARNI (n=639) | P | |
|---|---|---|---|---|
| Female sex | 335 (30.4) | 123 (27.3) | 207 (32.4) | .074 |
| Age, y | 75.0 [68.0-81.0] | 74 [66.0-80.0] | 75.7 [69.0-82.0] | <.001* |
| BMI, kg/m2 | 26.5 [23.9-29.5] | 26.2 [23.8-29.7] | 26.6 [24.0-29.4] | .504 |
| Diabetes | 368 (34.9) | 149 (35) | 218 (34.9) | .970 |
| Diabetes on insulin | 129 (30.7) | 54 (36) | 74 (27.5) | .070 |
| Hypertension | 776 (73.6) | 304 (71) | 471 (75.5) | .107 |
| Dyslipidemia | 615 (58.3) | 251 (58.6) | 363 (58.2) | .879 |
| Smoker | 298 (28.4) | 101 (23.7) | 197 (31.7) | .005* |
| Dialysis | 27 (2.6) | 10 (2.4) | 17 (2.8) | .698 |
| NYHA III-IV | 909 (87.2) | 366 (86.3) | 541 (87.7) | .519 |
| Ischemic heart disease | 557 (53.3) | 233 (55.3) | 323 (51.8) | .266 |
| Previous PCI | 363 (34.8) | 157 (37) | 206 (33.3) | .219 |
| Previous CABG | 162 (15.5) | 73 (17.2) | 88 (14.2) | .190 |
| Extracoronary arteriopathy | 154 (14.7) | 59 (13.8) | 94 (15.2) | .555 |
| Atrial fibrillation | 656 (62.5) | 240 (56.6) | 414 (66.5) | .001* |
| COPD | 246 (23.5) | 102 (24) | 144 (23.2) | .761 |
| Previous permanent pacemaker | 138 (13.2) | 55 (12.9) | 82 (13.2) | .909 |
| Stroke | 112 (10.7) | 35 (8.2) | 77 (12.4) | .033* |
| Prior TAVI | 33 (3.2) | 14 (3.3) | 19 (3.1) | .846 |
| Critical preoperative status | 55 (5.3) | 19 (4.6) | 36 (5.9) | .366 |
| EuroSCORE II, % | 7.4 [5.3-12.1] | 7.9 [5.6-12.0] | 7 [5.2-12.2] | .120 |
| STS score, % | 5.6 [4.1-7.8] | 5.6 [4.2-8.0] | 5.6 [4.1-7.8] | .780 |
| Urgent procedure | 72 (7.1) | 15 (4.4) | 49 (8.5) | .019* |
| Cardiac resynchronization therapy | 126 (12.0) | 48 (11.3) | 77 (12.4) | .585 |
| Frailty | 154 (15.1) | 50 (11.8) | 104 (17.4) | .013* |
| ACE inhibitors | 418 (43.4) | 221 (55.1) | 206 (35.6) | .001* |
| ARB | 283 (29.5) | 96 (25.3) | 187 (32.4) | .020* |
| Loop diuretics | 919 (95.1) | 366 (95.6) | 552 (94.8) | .613 |
| MRA | 503 (52.2) | 221 (55.4) | 292 (50.3) | .120 |
| Hydralazine+nitrates | 162 (17.4) | 86 (22.5) | 76 (13.9) | <.001* |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; ARNI; angiotensin receptor-neprilysin inhibitors; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PCI, percutaneous coronary interventions; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Data are expressed as No. (%) or median [interquartile range].
Echocardiographic features of the overall study population according to pre- or post-ARNI era
| Overall study population (N=1089) | Pre-ARNI (n=450) | Post-ARNI(n=639) | P | |
|---|---|---|---|---|
| Preprocedural features | ||||
| LVEF, % | 36 [30.0-52.5] | 35 [28.0-47.0] | 38 [30.0-55.0] | .011* |
| LVEDD, mm | 61.0 [55.0-68.0] | 62 [56.5-69.0] | 60 [55.0-68.0] | .021* |
| LVESD, mm | 50.0 [38.0-58.0] | 50 [38.0-58.0] | 48 [37.0-59.0] | .710 |
| LVEDV, mL | 153.0[106.0-195.6] | 157 [104.9-204.5] | 148.7 [106.0-190.0] | .102 |
| LVESV, mL | 90.0 [56.8-139.0] | 100 [62.2-146.0] | 82 [51.0-129.5] | .025* |
| PASP, mmHg | 52.050.0 [40.0-60.0] | 53 [45.0-62.0] | 50 [40.0-60.0] | .124 |
| TAPSE, mm | 14.616.0 [12.0-20.0] | 15 [8.0-19.0] | 17 [13.0-20.0] | .003* |
| Regurgitant volume, mL/beat | 59 [47.0-70.0] | 60 [49.0-74.0] | 57 [46.0-69.4] | .183 |
| Vena contracta, mm | 7.0 [6.0-8.0] | 7 [6.0-8.0] | 7.1 [6.0-8.0] | .894 |
| Annulus bicomissural diameter, mm | 37.0 [33.0-40.0] | 36 [31.0-40.0] | 37 [22.0-40.0] | .389 |
| Mitral gradient, mmHg | 1.0 [1.0-2-0] | 1 [1.0-2.0] | 1 [1.0-2.0] | .882 |
| Flail | 131 (20.4) | 50 (20.6) | 81 (20.3) | .930 |
| Annulus calcification | 130 (20.6) | 51 (19.2) | 79 (21.5) | .480 |
| TR grade 3-4 | 289 (32.8) | 101 (28.0) | 188 (36.2) | .011* |
| Mitral regurgitation grade 3-4 | 1089 (100.0) | 450 (100) | 639 (100) | .999 |
| LA mean pressure, mmHg | 18 [13.0-23.0] | 18 [14.0-23.0] | 18 [13.0-23.0] | .813 |
| Postprocedural features | ||||
| Mitral regurgitation grade 3-4 | 50 (5.0) | 22 (5.3) | 28 (4.9) | .776 |
| PASP, mmHg | 41.0 [33.5-50.0] | 46 [29.0-60.0] | 40 [34.5-50.0] | .753 |
| Mitral gradient, mmHg | 3.0 [2.0-4.0] | 3 [2.0-4.0] | 3 [2.0-4.0] | .009* |
| LA mean pressure, mmHg | 13.0 [9.0-17-0] | 11.5 [7.0-19.0] | 13 [9.0-17.0] | .880 |
ARNI; angiotensin receptor-neprilysin inhibitors; LA, left atrial; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volumen; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Data are expressed as No. (%) or median [interquartile range].
Mean value for pre-ARNI group 3.3±1.6mmHg vs mean value for post-ARNI group 3±1.5mmHg
The main baseline clinical characteristics of the study population are shown in table 1. Most TEER implantations were performed in male patients before (72.7%) and during the ARNI era (67.6%). Although patients who underwent TEER after the introduction of ARNIs had similar surgical risk, they were older (75.7 [69.0-82.0] vs 74.0 [66.0-80.0] years, P <.001), with a higher prevalence of atrial fibrillation (66.5% vs 56.6%, P=.001), prior stroke (12.4% vs 8.2%, P=.03), and frailty, particularly due to poor mobility (17.4% vs 11.8%, P=.010). Regarding baseline treatment, there were no differences in the rate of cardiac resynchronization therapy (11.3% vs 12.4%, P=.59) and patients who underwent TEER before the ARNI era more often took angiotensin-converting enzyme inhibitors (55.1% vs 35.6%, P <.001) with no other differences regarding medical management.
The main pre- and postprocedural echocardiographic characteristics of the study population are shown in table 2. Differences were found in left ventricular dimensions with larger left ventricular end-diastolic diameter and left ventricular end-systolic volume in patients undergoing TEER before the ARNI era. Biventricular systolic function was poorer in patients undergoing TEER before the ARNI era (left ventricular ejection fraction 35% [28.0-47.0] vs 38% [30.0-55.0], P=.011) and tricuspid annular plane systolic excursion of 15mm [8.0-19.0] vs 17mm [13.0-20.0], P=.003). Echocardiographic criteria to quantify severity of MR where similar between groups.
Comparison of patients before and after angiotensin receptor-neprilysin inhibitors era: procedural, in-hospital, and long-term outcomesThe main procedural and in-hospital outcomes are shown in table 3. Technical success was achieved in 96.7% of the procedures with no differences between groups. No differences were detected in the complication rate during the procedure but patients in the first term more often required the use of inotropes (13.4% vs 7.2%, P <.001) and intra-aortic balloon pump (7% vs 1.3%, P <.001) during the procedure. There were no significant differences in the degree of MR postprocedure between groups (P=.776). No deaths occurred intraprocedurally and the rate of in-hospital mortality was 14 (3.4%) vs 17 (3.1%), P=.775.
Procedural, in-hospital and long-term outcomes of the overall study population according to pre- or post-ARNI era
| Overall study population(N=1089) | Pre-ARNI (n=450) | Post-ARNI (n=639) | P | |
|---|---|---|---|---|
| Procedural outcomes | ||||
| Procedural success | 914 (94.1) | 395 (93.6) | 519 (94.5) | .540 |
| Technical success | 990 (96.7) | 410 (96.5) | 580 (96.8) | .753 |
| Procedural duration, min | 142.4±31.2 | 151.3±34.6 | 137.2±29.8 | .075 |
| No. of clips | 1 [1-2] | 1 [1-2] | 1 [1-3] | .219 |
| Catheter thrombosis | 5 (0.5) | 3 (0.7) | 2 (0.4) | .655 |
| Cordal rupture | 10 (1.0) | 7 (1.7) | 3 (0.5) | .103 |
| Cordal entrapment | 6 (0.6) | 4 (1) | 2 (0.4) | .240 |
| Procedure inotropes | 99 (9.7) | 56 (13.4) | 43 (7.2) | .001* |
| Procedure IABP | 36 (3.7) | 29 (7) | 7 (1.3) | .001* |
| In-hospital outcomes | ||||
| Mortality | 24 (2.2) | 14 (3.1) | 10 (1.6) | .087 |
| Hematoma | 66 (6.7) | 28 (6.8) | 38 (6.7) | .912 |
| Pseudoaneurysm | 17 (1.7) | 7 (1.7) | 10 (1.8) | .958 |
| AV fistula | 13 (1.3) | 5 (1.2) | 8 (1.4) | .806 |
| Vascular surgery | 5 (0.5) | 3 (0.7) | 2 (0.4) | .654 |
| Bleeding BARC 3 | 21 (1.9) | 11 (4.4) | 10 (3.4) | .546 |
| Transfusion | 67 (6.9) | 31 (7.6) | 6 (6.3) | .447 |
| Long-term outcomes | ||||
| Mortality at 30 d | 21 (1.9) | 10 (2.2) | 11 (1.7) | .554 |
| Mortality at 1 y | 108 (9.8) | 54 (12) | 54 (8.5) | .054 |
| Mortality at 2 y | 146 (13.3) | 78 (17.3) | 68 (10.6) | .001* |
| NYHA III-IV at 2 y | 286 (37.8) | 169 (44.5) | 117 (31.1) | <.001* |
| HF readmission at 2 y | 231 (21) | 125 (27.8) | 106 (16.6) | <.001* |
| Mitral regurgitation 3-4 | 110 (15.5) | 65 (17.6) | 45 (13.3) | .111 |
| Mortality+HF readmission at 2 y | 300 (27.2) | 162 (36) | 138 (21.6) | <.001* |
ARNI; angiotensin receptor-neprilysin inhibitors; AV, arterial-venous; BARC, Bleeding Academic Research Consortium; HF, heart failure; IABP, intra-aortic balloon pump; NYHA, New York Heart Association.
Data are expressed as No. (%) or median [interquartile range].
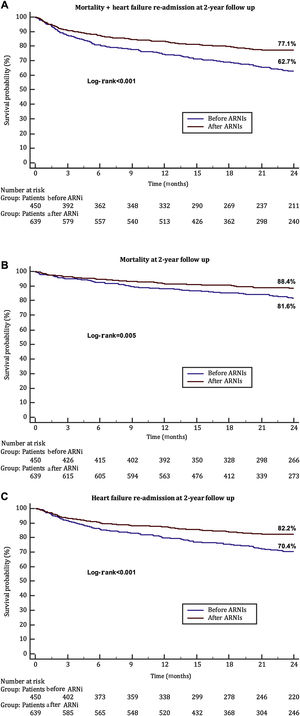
Mortality at 30 days and 1 year were similar between groups (2.2% vs 1.7%, P=.999 and 12% vs 8.5%, P=.054, respectively). Severe symptoms (defined as NYHA III-IV) during the follow-up were less common in the ARNI era: 117 (31.1%) vs 169 (44.5%), P <.001. Mortality, readmission due to HF and the combined endpoint of HF rehospitalization and mortality at 2 years of follow-up were lower in the ARNI era (17.3% vs 10.6%, P <.001; 27.8% vs 16.6%, P <.001 and 36% vs 21.6%, P <.001, respectively) (figure 1). Residual MR at 2 years of follow-up showed no differences between the 2 temporal cohorts as depicted in .
Combined endpoint of death and heart failure readmission at 2 years of follow-up (A) and each component (B: death; C: heart failure readmission) in patients undergoing edge-to-edge treatment according to time period (before or after ARNI availability). ARNIs, angiotensin receptor-neprilysin inhibitors.
The main baseline clinical characteristics of this more recent subgroup (n=639, 58.6%), as well as the main differences according to the use of ARNIs is shown in . Patients receiving ARNIs (n=52, 8.1%) were younger (73 years [65.0-79.5] vs 76 years [69.8-82.0], P=.037) and more often had cardiac resynchronization therapy devices (21.2% vs 11.6%, P=.045). There were no differences in the rate of prior atrial fibrillation or stroke. In addition, EuroSCORE II, the Society of Thoracic Surgeons risk score and frailty were comparable. Regarding medical treatment, there was a trend to greater use of mineralocorticoid receptor inhibitors in patients receiving ARNIs (62% vs 49.2%, P=.082), with no other differences.
Baseline echocardiographic parameters were similar, including left ventricular dimensions and function, right ventricular function, and quantitative evaluation of MR severity, as shown in . There were no differences in the rate of complications during the procedure or in the use of inotropes or intra-aortic balloon pump. The degree of MR postprocedure between groups was comparable (P=.999) (). Mortality at 30 days (no-ARNIs: 1.9% vs ARNIs: 0%, P=.999), 1 year (no-ARNIs: 8.7% vs ARNIs: 5.8%, P=.609), and 2 years (no-ARNIs: 11.1% vs ARNIs: 5.8%, P=.235) was numerically lower among patients on ARNIs, although this difference was nonsignificant. Patients under ARNIs developed severe symptoms less often during follow-up (NYHA III-IV) 4 (15.4%) vs 113 (32.3%), P=.073 and the use of ARNIs was independently associated with a lower risk of the combined event at 2-year follow-up (odds ratio, 0.369; 95% confidence interval [95%CI], 0.137-0.992, P=.048) (table 4) (); finally, despite similar procedural results in terms of residual MR, there was a lower rate of recurrent MR at 2 years in patients taking ARNIs (1 (1.9%) vs 44 (14.3%), P=.011) (figure 2).
Independent predictors of mortality and heart failure rehospitalization in the ARNI era
| Variables | Univariate | Multivariatea | ||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| ARNI intake | 0.450 (0.188-1.076) | .073 | 0.369 (0.137-0.992) | .048b |
| NYHA class III/IV | 2.634 (1.233-5.627) | .012 | 3.745 (1.439-9.748) | .007b |
| Extracoronary arteriopathy | 1.973 (1.221-3.189) | .006 | 2.048 (1.165-3.602) | .013b |
| Chronic obstructive pulmonary disease | 1.526 (0.995-2.342) | .053 | 1.827 (1.104-3.022) | .019b |
| Previous cardiac artery bypass graft | 1.627 (0.983-2.694) | .058 | - | - |
| Frailty | 1.799 (1.128-2.871) | .014 | - | - |
| Age | 1.031 (1.009-1.054) | .005 | 1.038 (1.011-1.065) | .005b |
| EuroSCORE II | 1.045 (1.033-1.088) | .035 | - | - |
| Creatinine | 1.269 (1.039-1.549) | .019 | 1.295 (1.037-1.617) | .022b |
| Hemoglobin | 0.857 (0.749-0.979) | .024 | - | - |
| Cardiac resynchronization therapy | 1.298 (0.749-2.247) | .367 | ||
95%CI, 95% confidence interval; ARNIs, angiotensin-converting enzyme inhibitors; NYHA, New York Heart Association; OR, odds ratio.
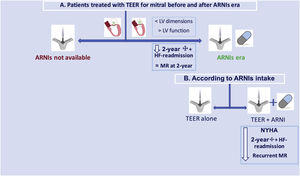
According to NYHA class, left ventricular ejection fraction and renal function, a total of 143 patients (22.4% of the patients in the ARNI era) had an indication for ARNI prescription but did not receive this drug. Compared with patients receiving ARNIs (8.1%), their mortality rate at 2 years was similar (12.6% vs 5.8%, P=.174), but the combined endpoint at 2 years was significantly higher (25.9% vs 11.5%, P=.033) and NYHA class III-IV symptoms was also worse (37.2% vs 15.4%, P=.035). A summary of the main findings is depicted in the figure 3.
Central illustration. Summary of the study including patients before and once sacubitril-valsartan (ARNIs) was available and according to the intake of the drug. ARNIs, angiotensin receptor-neprilysin inhibitors; HF, heart failure; MR, mitral regurgitation; NYHA, New York Heart Association; TEER, transcatheter edge-to-edge repair.
After more than a decade of treating functional MR with edge-to-edge techniques, the profile of patients considered optimal candidates for this treatment has become clearer due to both research that has improved patient selection and the learning curve in technical aspects. In addition, new oral drugs that have improved the prognosis of HF patients have influenced the stage at which patients are referred for percutaneous treatment since the invasive approach is reserved only for those who remain symptomatic under optimal medical therapy. The main findings of this study, which explored the simultaneous influence of better candidate selection, the learning curve, and the adoption of ARNIs as the standard of care in symptomatic HF patients are as follows: a) patients selected for edge-to-edge therapy after 2017, when ARNIs became available in our setting, had better ventricular function associated with clinical experience in the selection process and likely influenced by the main findings of the COAPT trial8; b) in this “second generation” of patients treated with edge-to-edge therapy and despite their older age, greater number of comorbidities and more frequent need of urgent procedures, survival and rehospitalization rates at 2 year of follow-up were significantly improved; c) after ARNIs became available in our setting, the introduction of this therapy has been low with up to 30% of patients having an indication but only 8.1% of them receiving it; importantly, those who underwent edge-to-edge therapy while receiving ARNIs had a lower rate of the combined endpoint of mortality and HF rehospitalization, better NYHA class, and a lower rate of recurrent MR at 2 year of follow-up.
Changes in the clinical profile of patients receiving mitral edge-to-edge therapyThe results of pivotal trials suggested a potential but inconclusive beneficial impact of TEER.9,10 Due to the shortcomings of these trials, in recent years the landmark COAPT8 and MITRA-FR11 trials published their results almost simultaneously. Both trials showed contradictory results to those of the other, with the COAPT trial clearly favoring the use of the TEER strategy. The notable exclusion criteria of this trial were very low ejection fraction (< 20%), very dilated left ventricle (> 70 mm), right-sided HF, advanced lung disease, and other severe heart valve disease. Importantly, 95% of patients had ≤ 2+MR at 12 months, demonstrating the effectiveness of this treatment.8 Of note, patients in our research, particularly in the ARNI era, exhibited clinical features similar to those in the COAPT trial regarding left ventricular volumes and technical and procedural success rates. This trend to a COAPT-like profile accounts for an improvement in the selection of candidates suitable for mitral valve repair and likely had a positive effect on procedural results and prognosis, as mortality in our study was even lower than that observed in the COAPT trial; indeed, the difference in 1-year mortality in the second period of our study was almost 4% but this was not statistically significant.12 The findings of this study represent the cornerstone for better results of this therapy alone, even despite the use of novel pharmacological therapies.
Clinical impact of angiotensin receptor-neprilysin inhibitors in patients with symptomatic heart failure and mitral regurgitationRecently, the PRIME trial (Pharmacological Reduction of Functional, Ischemic Mitral Regurgitation) was one of the first to explore whether medical treatment can reduce functional MR, comparing sacubitril/valsartan vs valsartan alone.13 The study proved that there was a significant decrease of the effective orifice area of MR following 12 months of treatment with ARNIs; of note, changes in left ventricular volumes were limited and showed no differences between the valsartan and sacubitril/valsartan groups. Left atrial size was significantly decreased in the sacubitril/valsartan group comparison with that in the valsartan group, but there were no significant differences in other echocardiographic endpoints, including ejection fraction.
It is well known that MR severity is strongly associated with all-cause mortality and HF hospitalizations.14–17 However, left ventricular remodeling was limited following ARNIs administration and the MR reduction might be mainly explained by a significantly greater reduction in arterial impedance (afterload) in the sacubitril/valsartan group. This is highly important, given that according to MITRA-FR and COAPT findings, left ventricular dimensions and function determine post-TEER prognosis. Although guideline-directed medical therapy for HF is the first-line treatment for patients with secondary functional MR, this strategy is usually insufficient. Better characterization and stratification of the secondary MR population may allow a distinction between those patients who may obtain prognostic benefit and those who will derive symptom relief and reduced need for hospitalization only. In practice, this may not be simple, since patient-level analysis failed to identify any combination of echocardiographic parameters associated with clinical benefit following intervention in these trials (including those with disproportionate MR). Since the safety and usefulness of TEER have now been proven in selected patients, the potential for intervention earlier in the natural history of the disease to prevent irreversible LV remodeling and systolic impairment will need to be rigorously evaluated in future studies.
Even though few patients took ARNIs in our study cohort, there was a clear reduction in the composite primary endpoint of hospitalization for HF and death at 2 years and a reduced rate of recurrent MR. This could represent a synergic effect of 2 different mechanisms to treat functional MR, a mechanical one (TEER) and another mediated by remodeling pathways (ARNIs).18 Indeed, in our analysis, ARNIs alone reduced the combined endpoint and symptoms in patients prescribed these drugs compared with patients not taking them despite having a clear indication for this therapy (table 5).
Comparison of patients on ARNIs with patients with an indication who were not taking this medication
| Treated with ARNI (n=52) | ARNIs indicated but not prescribed (n=143) | P | |
|---|---|---|---|
| Long-term outcomes | |||
| Mortality at 30 d | 0 (0.0) | 3 (2.1) | .566 |
| Mortality at 1 y | 3 (5.8) | 16 (11.2) | .259 |
| Mortality at 2 y | 3 (5.8) | 18 (12.6) | .174 |
| NYHA III-IV at 2 y | 4 (15.4) | 35 (37.2) | .035* |
| HF readmission at 2 y | 5 (9.6) | 30 (21.0) | .067 |
| Mitral regurgitation 3-4 | 1 (3.1) | 11 (13.1) | .175 |
| Mortality+HF readmission at 2 y | 6 (11.5) | 37 (25.9) | .033* |
ARNIs, angiotensin-converting enzyme inhibitors; HF, heart failure; NYHA, New York Heart Association.
Data are expressed as No. (%).
ARNI indication in patients fulfilling 3 criteria: NYHA> 2, LVEF <40%, and creatinine <1.5.
Currently, patients who are referred for TEER are highly complex, with multiple comorbidities and often at a late stage of their disease.1 Whether current timing is appropriate or not is under debate; although the use of ARNIs and other new drugs such as sodium-glucose cotransporter-2 inhibitors may improve the prognosis of HF patients, there is a risk of excessive delay in referral for TEER, since left ventricular deterioration dramatically affects prognosis. Therefore, early implementation of TEER in the presence of persistent symptoms and MR following ARNI prescription might be crucial to achieve the above-mentioned synergic effect.19 In our study population, the median time between ARNI prescription and the TEER procedure was 6 months (range 1-13 months), which lies within expert recommendation20; even so, there is sufficient evidence showing that patients with worse left ventricular function and size have worse prognosis,8,11,21,22 and, in them, the sequential early implementation of both strategies could have a complementary effect and potentially increase the number of candidates to TEER including certain MITRA-FR-like cases.
LimitationsThe main limitations of this study include the retrospective analysis of the data; although we prospectively collected information and carefully reviewed medication, we did not gather information on the continuation of ARNIs in patients prescribed this medication, potential crossover treatments, or dosage changes. In addition, patients were included at the discretion of each institution and the lack of monitoring led to close to 2% of patients being lost to follow-up. No adjustment was made when we compared patients with and without ARNIs due to the relatively reduced number of patients receiving this drug although logistic regression helped to correct confounders. The time from ARNI prescription to TEER was not compared due to the reduced sample size.
CONCLUSIONSPatients treated with TEER in recent years had significantly improved survival and HF hospitalization rates at 2 years of follow-up. Better patient selection for TEER played an important role in this improvement in outcomes. However, patients prescribed ARNIs had better symptoms relief, a further reduction in the rate of MR recurrence in the long-term, and a reduction in the combined endpoint of mortality and readmissions, particularly compared with those with an indication for ARNIs but not prescribed these drugs in this period. This reinforces the need for greater prescription of ARNIs in this population.
- –
TEER reduces MR in patients with HF and secondary MR; the COAPT trial suggested that, in patients with a smaller left ventricle and better left ventricular ejection fraction, TEER was associated with lower mortality than optimal medical therapy. However, the definition of optimal medical therapy also changed after that study to include new pharmacotherapy such as ARNIs. It is unknown whether the extended pharmacotherapy spectrum on the treatment of patients with secondary MR and HF influences the timeline selection of candidates and/or the outcomes of patients undergoing TEER.
- –
Patients treated with TEER in recent years had significantly improved survival and HF hospitalization rates at 2 years of follow-up. Better patient selection for TEER played an important role for this improvement in outcomes. However, use of ARNIs was also associated with better symptoms relief, a further reduction in the rate of MR recurrence in the long-term, and a reduction in the combined endpoint of mortality and readmissions. This reinforces the need for greater prescription of ARNIs in this population.
None.
AUTHORS’ CONTRIBUTIONSAll authors have significantly contributed to the manuscript and have approved the final version.
CONFLICTS OF INTERESTI. Pascual, D. Arzamendi, R. Estevez-Loureiro, and X. Freixa are proctors for Abbott Vascular. J. Sanchis is editor-in-chief and P. Avanzas is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. The remaining authors have nothing to disclose.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.02.001
Roman Arnold (CIBERCV, Departamento de Cardiología, Hospital Clínico Universitario de Valladolid, Valladolid, Spain), Víctor Manuel Becerra-Muñoz, and Antonio Jesús Domínguez-Franco (CIBERCV, Departamento de Cardiología, Hospital Virgen de la Victoria, Málaga, Spain), Javier Gualis, and Armando Pérez de Prado (CIBERCV, Departamento de Cardiología, Hospital Clínico Universitario, León, Spain), César Moris (Departamento de Cardiología, Hospital Universitario Central de Asturias, Oviedo, Asturias, Spain), Manel Sabate (Departamento de Cardiología, Hospital Clinic, Barcelona, Spain), Rosana Hernández Antolín and Luisa Salido (Departamento de Cardiología, Hospital Ramón y Cajal, Madrid, Spain), and Javier Goicolea (Departamento de Cardiología, Hospital Universitario Puerta de Hierro, Majadahonda, Madrid, Spain).