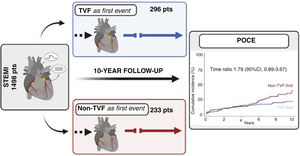
After ST-segment myocardial infarction (STEMI), the impact of different adverse events on prognosis remains unknown. We aimed to assess very long-term predictors of patient-oriented composite endpoints (POCE) and investigate whether the occurrence of target vessel failure (TVF) vs a non-TVF event as the first event could potentially influence subsequent outcomes.
MethodsThe EXAMINATION-EXTEND trial randomized STEMI patients to receive either an everolimus-eluting stent or a bare-metal stent. The follow-up period was 10 years. Predictors of POCE (a composite of all-cause death, any myocardial infarction, or any revascularization) were evaluated in the overall study population. The patients were stratified based on the type of first event (TVF-first vs non–TVF-first) and were compared in terms of subsequent POCE. TVF was defined as a composite of cardiac death, TV myocardial infarction, or TV revascularization.
ResultsOut of the 1498 enrolled patients, 529 (35.3%) experienced a POCE during the 10-year follow-up. Independent predictors of POCE were age, diabetes mellitus, previous myocardial infarction, peripheral arterial disease, and multivessel coronary disease. The first event was a TVF in 296 patients and was a non-TVF in 233 patients. No significant differences were observed between TVF-first and non–TVF-first patients in terms of subsequent POCE (21.7% vs 39.3%, time ratio 1.79; 95%CI, 0.87-3.67;P=.12) or its individual components.
ConclusionsAt the 10-year follow-up, approximately one-third of STEMI patients had experienced at least 1 POCE. Independent predictors of these events were age, diabetes, and more extensive atherosclerotic disease. The occurrence of a TVF or a non-TVF as the first event did not seem to influence subsequent outcomes. Trial registration number: NCT04462315.
Keywords
ST-segment elevation myocardial infarction (STEMI) patients are at high risk for subsequent cardiovascular events.1 Their outcomes may vary significantly, with some experiencing event-free periods lasting years while others have recurrent events. Due to improvements in procedural aspects and continuous advancements in pharmacotherapy, the proportion of STEMI patients with event-free follow-up has increased over the years. However, data on very long-term follow-up are scarce or focus on mortality rates.2–5 Additionally, little is known about the impact of different types of adverse events on subsequent outcomes. Previous data from the HORIZON-AMI trial showed that target vessel revascularization (TVR) was associated with an increased risk of subsequent myocardial infarction,6 while reinfarctions increased the risk of death.7 Nevertheless, it remains unknown whether the time of occurrence of such events and their association with the culprit vessel can determine the nature of future outcomes.
We aimed to assess the very long-term predictors of a patient-oriented composite endpoint (POCE) and determine whether having a target vessel failure (TVF) vs a non-TVF as the first event would influence subsequent outcomes in the STEMI population from the EXAMINATION-EXTEND trial.
METHODSStudy populationThe EXAMINATION-EXTEND trial, a multicenter, prospective, controlled trial, randomly assigned 1498 STEMI patients to receive either an everolimus-eluting stent or a bare-metal stent. The inclusion and exclusion criteria, as well as the clinical outcomes at the 5-year follow-up, have been previously reported.8 The EXAMINATION-EXTEND trial was subsequently reinitiated as an EXAMINATION study (NCT04462315) to focus on 10-year outcomes. This follow-up study maintained the same methodology and event adjudication as the original trial. A complete clinical follow-up was obtained for 1427 patients (95.3%). Detailed information on the study and the 10-year follow-up results have been previously reported.9–11
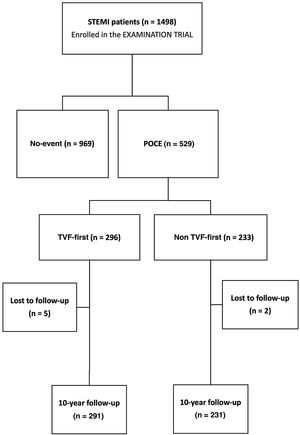
This study is a post-hoc analysis conducted to evaluate the 10-year predictors of POCE in the overall population of the trial. Additionally, patients were stratified based on the type of first event, either TVF-first or non–TVF-first, and subsequently compared in terms of subsequent POCE (figure 1). In this analysis, TVF was defined as a composite outcome consisting of cardiac death, target vessel myocardial infarction, and target vessel revascularization (TVR).12 Non-target vessel failure (non-TVF) encompassed noncardiac death, nontarget vessel myocardial infarction, and nontarget vessel revascularization.
Flow chart of the EXAMINATION trial, which enrolled 1498 patients. Patients were stratified first according to the occurrence of any POCE during the 10-year follow-up. Then, the 529 patients who experienced at least 1 event where further stratified according to the type of first event into 2 groups, TVF-first vs non–TVF-first, with comparison of subsequent outcomes up to 10 years. Loses to follow-up in the TVF-first and non–TVF-first groups were 1.7% and 0.9%, respectively. POCE, patient-oriented composite endpoint; STEMI, ST elevation myocardial infarction; TVF, target vessel failure.
The primary endpoint of this study was defined as a POCE consisting of all-cause death, any myocardial infarction, or any revascularization. Secondary endpoints included the individual components of the POCE, as well as cardiac death, target vessel myocardial infarction, target lesion or TVR, and stent thrombosis (definite/probable). All events were adjudicted by an independent clinical events committee, specifically the Barcicore Lab in Spain, based on the definitions provided by the Academic Research Consortium.13
Statistical analysisQuantitative variables are expressed as either mean and standard deviation or median and interquartile range, depending on their distribution. Statistical comparisons between groups were performed using either the Student t-test or the Mann–Whitney U test, as appropriate. Categorical variables are expressed as numbers and percentages and were compared using either the chi-square test or Fisher exact test, as appropriate. To assess the independent correlates of the first occurrence of POCE and the independent predictors of TVF/non-TVF-first events, a Cox regression model was used. All variables that achieved a P value of <.10 in the univariate analysis were included in the Cox model.
Long-term outcomes were compared between patients according to the nature of their first event (TVF-first vs non–TVF-first). The follow-up time for TVF-first and non–TVF-first patients began from the date of their first event occurrence. Any subsequent recurrent event of each type during the remaining follow-up period was included in the analysis, as required for time-to-event analysis. The Kaplan-Meier method was used to estimate event rates. The cumulative incidence of the primary and secondary endpoints was calculated, taking into account any death as a competing risk.14 Since the proportional hazard assumption for the primary endpoint, tested with Schoenfeld residuals, was not met (P <.05), both the accelerated time failure model and the difference in restricted mean survival time15 were used as the primary analyses to compare the effect of TVF-first or non–TVF-first on subsequent outcomes. The accelerated time failure model provided a Time Ratio, which describes the estimated delay until an event occurs in one group compared with the other. The restricted mean survival time represents the mean time free from an outcome event throughout the follow-up. Additionally, a landmark analysis was conducted to better describe the change in the risk of the primary and secondary endpoints over time. In this exploratory analysis, hazard ratios were estimated between the 2 groups within 2 intervals: 0 to 5 years and 5 to 10 years, during which proportional hazards were preserved. The hazard ratio, time ratio, and difference in restricted mean survival time are reported with associated 95% confidence intervals and P values. Statistical significance was considered for a 2-tailed P value <.05. Statistical analyses were performed using STATA, Version 14.1 (StataCorp LLC, United States.
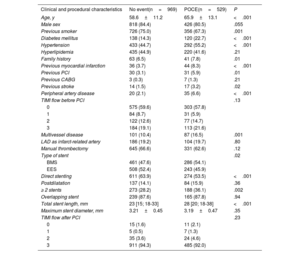
RESULTSOverall patient populationOut of the 1498 STEMI patients enrolled in the EXAMINATION-EXTEND study, 969 (64.7%) had an event-free 10-year follow-up, while 529 (35.3%) experienced a POCE. The clinical and procedural characteristics of these 2 groups are shown in table 1. Compared with patients with an event-free follow-up, those who experienced a POCE were older and had a higher prevalence of smoking, diabetes mellitus, hypertension, a family history of coronary artery disease, as well as a history of previous myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft, stroke, and peripheral artery disease. In terms of procedural data, patients with POCE had an increased prevalence of multivessel disease, and more frequently received bare-metal stents and direct stenting, with longer stent length and a higher number of stents.
Baseline clinical and procedural characteristics of patients with and without a 10-year POCE
| Clinical and procedural characteristics | No event(n=969) | POCE(n=529) | P |
|---|---|---|---|
| Age, y | 58.6±11.2 | 65.9±13.1 | <.001 |
| Male sex | 818 (84.4) | 426 (80.5) | .055 |
| Previous smoker | 726 (75.0) | 356 (67.3) | .001 |
| Diabetes mellitus | 138 (14.3) | 120 (22.7) | <.001 |
| Hypertension | 433 (44.7) | 292 (55.2) | <.001 |
| Hyperlipidemia | 435 (44.9) | 220 (41.6) | .21 |
| Family history | 63 (6.5) | 41 (7.8) | .01 |
| Previous myocardial infarction | 36 (3.7) | 44 (8.3) | <.001 |
| Previous PCI | 30 (3.1) | 31 (5.9) | .01 |
| Previous CABG | 3 (0.3) | 7 (1.3) | .21 |
| Previous stroke | 14 (1.5) | 17 (3.2) | .02 |
| Peripheral artery disease | 20 (2.1) | 35 (6.6) | <.001 |
| TIMI flow before PCI | .13 | ||
| 0 | 575 (59.6) | 303 (57.8) | |
| 1 | 84 (8.7) | 31 (5.9) | |
| 2 | 122 (12.6) | 77 (14.7) | |
| 3 | 184 (19.1) | 113 (21.6) | |
| Multivessel disease | 101 (10.4) | 87 (16.5) | .001 |
| LAD as infarct-related artery | 186 (19.2) | 104 (19.7) | .80 |
| Manual thrombectomy | 645 (66.6) | 331 (62.6) | .12 |
| Type of stent | .02 | ||
| BMS | 461 (47.6) | 286 (54.1) | |
| EES | 508 (52.4) | 243 (45.9) | |
| Direct stenting | 611 (63.9) | 274 (53.5) | <.001 |
| Postdilatation | 137 (14.1) | 84 (15.9) | .36 |
| ≥ 2 stents | 273 (28.2) | 188 (36.1) | .002 |
| Overlapping stent | 239 (87.6) | 165 (87.8) | .94 |
| Total stent length, mm | 23 [15; 18-33] | 28 [20; 18-38] | <.001 |
| Maximum stent diameter, mm | 3.21±0.45 | 3.19±0.47 | .35 |
| TIMI flow after PCI | .23 | ||
| 0 | 15 (1.6) | 11 (2.1) | |
| 1 | 5 (0.5) | 7 (1.3) | |
| 2 | 35 (3.6) | 24 (4.6) | |
| 3 | 911 (94.3) | 485 (92.0) |
BMS, bare-metal stent; CABG, coronary artery bypass graft; EES, everolimus-eluting stent; IQR, interquartile range; LAD, left anterior descending; PCI, percutaneous coronary intervention; TVF, target vessel failure.
Data are presented as No. (%), mean±standard deviation of median [interquartile range].
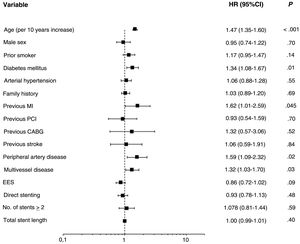
On multivariate analysis, the only independent predictors of POCE occurrence were age (hazard ratio [HR] 1.48, 95%CI: 1.34-1.63;P <.001), diabetes mellitus (HR 1.34, 95%CI, 1.08-1.66; P=.01), previous myocardial infarction (HR 1.66; 95%CI: 1.04-2.63,P=.03), peripheral artery disease (HR 1.58, 95%CI: 1.08-2.31; P=.02), and multivessel disease (HR 1.33; 95%CI; 1.04-1.70; P=.02) (figure 2).
Forest plot presenting the results of a multivariate analysis of factors associated with the risk of POCE in the overall population. The analysis was performed with a Cox regression model including all variables obtaining a P value of <.10 in the univariate analysis. independent predictors of 10-year POCE were age, diabetes mellitus, previous myocardial infarction, peripheral artery disease, and multivessel disease. CABG, coronary artery bypass graft; 95%CI, 95% confidence interval; EES, everolimus-eluting stent; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention.
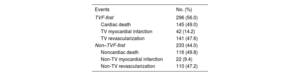
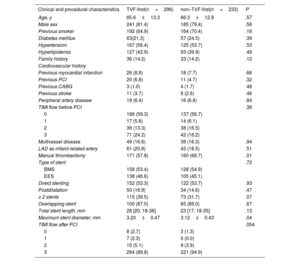
Among the 529 patients with POCE, the first event was a TVF in 296 and a non-TVF in 233 patients. The component of the TVF event was cardiac death in 49%, target vessel myocardial infarction in 14.2%, and TVR in 47.6%. In non-TVF, the component was noncardiac death in 44.1%, nontarget vessel myocardial infarction in 9.4%, and non-TVR in 47.2% (table 2). The median [interquartile range] time was 718 [70-1961] days to TVF-first and and 1518 [213-2495] days to non–TVF-first. Baseline and procedural characteristics between TVF-first and non–TVF-first groups differed only in manual thrombectomy use (P=.01), and the maximum diameter of the stent implanted in the culprit lesion (P=.04) (table 3), while no independent predictors were found in the multivariate analysis.
Type of events qualifying TVF-first and non–TVF-first groups
| Events | No. (%) |
|---|---|
| TVF-first | 296 (56.0) |
| Cardiac death | 145 (49.0) |
| TV myocardial infarction | 42 (14.2) |
| TV revascularization | 141 (47.6) |
| Non–TVF-first | 233 (44.0) |
| Noncardiac death | 116 (49.8) |
| Non-TV myocardial infarction | 22 (9.4) |
| Non-TV revascularization | 110 (47.2) |
POCE, patient-oriented composite endpoint; TV target vessel; TVF, target vessel failure.
Data are presented as No. (%).
Baseline clinical and procedural characteristics of TVF-first and non–TVF-first patients
| Clinical and procedural characteristics | TVF-first(n=296) | non–TVF-first(n=233) | P |
|---|---|---|---|
| Age, y | 65.6±13.3 | 66.3±12.8 | .57 |
| Male sex | 241 (81.4) | 185 (79.4) | .56 |
| Previous smoker | 192 (64.9) | 164 (70.4) | .18 |
| Diabetes mellitus | 63(21.3) | 57 (24.5) | .39 |
| Hypertension | 167 (56.4) | 125 (53.7) | .53 |
| Hyperlipidemia | 127 (42.9) | 93 (39.9) | .49 |
| Family history | 36 (14.2) | 33 (14.2) | .12 |
| Cardiovascular history | |||
| Previous myocardial infarction | 26 (8.8) | 18 (7.7) | .66 |
| Previous PCI | 20 (6.8) | 11 (4.7) | .32 |
| Previous CABG | 3 (1.0) | 4 (1.7) | .48 |
| Previous stroke | 11 (3.7) | 6 (2.6) | .46 |
| Peripheral artery disease | 19 (6.4) | 16 (6.9) | .84 |
| TIMI flow before PCI | .36 | ||
| 0 | 166 (59.3) | 137 (56.7) | |
| 1 | 17 (5.8) | 14 (6.1) | |
| 2 | 39 (13.3) | 38 (16.5) | |
| 3 | 71 (24.2) | 42 (18.2) | |
| Multivessel disease | 49 (16.6) | 38 (16.3) | .94 |
| LAD as infarct-related artery | 61 (20.8) | 43 (18.5) | .51 |
| Manual thrombectomy | 171 (57.8) | 160 (68.7) | .01 |
| Type of stent | .72 | ||
| BMS | 158 (53.4) | 128 (54.9) | |
| EES | 138 (46.6) | 105 (45.1) | |
| Direct stenting | 152 (53.3) | 122 (53.7) | .93 |
| Postdilatation | 50 (16.9) | 34 (14.6) | .47 |
| ≥ 2 stents | 115 (39.5) | 73 (31.7) | .07 |
| Overlapping stent | 100 (87.0) | 65 (89.0) | .67 |
| Total stent length, mm | 28 [20; 18-38] | 23 [17; 18-35] | .13 |
| Maximum stent diameter, mm | 3.20±0.47 | 3.12±0.43 | .04 |
| TIMI flow after PCI | .054 | ||
| 0 | 8 (2.7) | 3 (1.3) | |
| 1 | 7 (2.3) | 0 (0.0) | |
| 2 | 15 (5.1) | 9 (3.9) | |
| 3 | 264 (89.8) | 221 (94.9) |
BMS, bare-metal stent; CABG, coronary artery bypass graft; EES, everolimus-eluting stent; IQR, interquartile range; LAD, left anterior descending; PCI, percutaneous coronary intervention; TVF, target vessel failure.
The data are presented as No. (%), mean±standard deviation, or median [interquartile range].
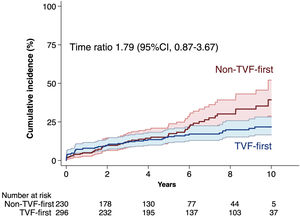
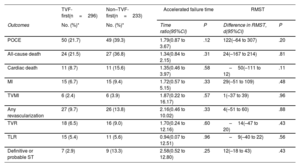
Fifty out of 296 patients in the TVF-first group and forty-nine out of 233 patients in the non–TVF-first group had a subsequent POCE during their remaining follow-up. According to the accelerated failure estimation model, there was no significant difference in the time to primary endpoint between TVF-first vs non–TVF-first (time ratio 1.79, 95%CI, 0.87-3.67;P=.12) (figure 3). The restricted mean survival time was 2852 days for the TVF-first group and 2731 days for the non–TVF-first group (difference, 121 days, 95%CI,−64 to 307; P=.20). Furthermore, as shown in table 4, the time to occurrence of any secondary outcome did not statistically differ between TVF-first and non–TVF-first patients.
Kaplan-Meier estimated cumulative incidence with 95% confidence intervals of subsequent POCE according to the type of first event: TVF-first vs non-TVF-first. Because of the nonproportionality of the hazards, the effect of TVF-first or non–TVF-first on subsequent POCE was evaluated with an accelerated time failure model providing a time ratio value that describes the estimated delay until the occurrence of an event one group compared with the other. CI, confidence interval; POCE, patient-oriented composite endpoint; TVF, target vessel failure.
Subsequent outcomes in TVF-first vs non–TVF-first patients
| TVF-first(n=296) | Non–TVF-first(n=233) | Accelerated failure time | RMST | |||
|---|---|---|---|---|---|---|
| Outcomes | No. (%)* | No. (%)* | Time ratio(95%CI) | P | Difference in RMST, d(95%CI) | P |
| POCE | 50 (21.7) | 49 (39.3) | 1.79(0.87 to 3.67) | .12 | 122(–64 to 307) | .20 |
| All-cause death | 24 (21.5) | 27 (36.8) | 1.34(0.84 to 2.15) | .31 | 24(–167 to 214) | .81 |
| Cardiac death | 11 (8.7) | 11 (15.6) | 1.35(0.46 to 3.97) | .58 | –50(–111 to 12) | .11 |
| MI | 15 (6.7) | 15 (9.4) | 1.72(0.57 to 5.15) | .33 | 29(–51 to 109) | .48 |
| TVMI | 6 (2.4) | 6 (3.9) | 1.87(0.22 to 16.17) | .57 | 1(–37 to 39) | .96 |
| Any revascularization | 27 (9.7) | 26 (13.8) | 2.16(0.46 to 10.02) | .33 | 4(–51 to 60) | .88 |
| TVR | 18 (6.5) | 16 (9.0) | 1.70(0.24 to 12.16) | .60 | –14(–47 to 20) | .43 |
| TLR | 15 (5.4) | 11 (5.6) | 0.94(0.07 to 12.51) | .96 | –9(–40 to 22) | .56 |
| Definitive or probable ST | 7 (2.9) | 9 (13.3) | 2.58(0.52 to 12.80) | .25 | 12(–18 to 43) | .43 |
95%CI, 95% confidence interval; HR, hazard ratio; MI, myocardial infarction; POCE, patient-oriented composite endpoint; ST, stent thrombosis; TVF, target vessel failure; TLR, target lesion revascularization; TVMI, target vessel myocardial infarction TVR, target vessel revascularization.
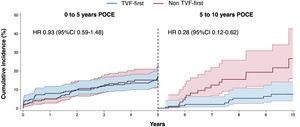
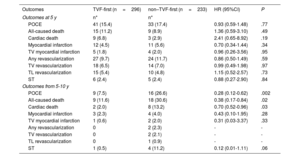
The risk of the primary endpoint varied over time, with no difference in the event rate between the 2 groups (TVF-first vs non-TVF-first) during the first 5 years of follow-up (HR, 0.93; 95%CI, 0.59-1.48; P=.77), while the TVF-first group was characterized by a lower risk of POCE (HR 0.28; 95%CI, 0.12-0.62; P=.002) from 5 to 10 years (figure 4). The reduced risk of POCE during the late follow-up of TVF-first patients was mainly driven by a lower rate of late mortality (table 5).
Landmark analysis showing the cumulative incidence of POCE within 2 intervals. Cumulative incidence curves with 95% confidence intervals (95%CI) estimated with the Kaplan-Meier method within 2 intervals: the first 5 years and from 5 to 10 years of follow-up. Hazard ratios (HR) with 95%CI were determined by Cox regression. POCE, patient-oriented composite endpoint; TVF, target vessel failure.
Outcomes in the landmark analysis
| Outcomes | TVF-first (n=296) | non–TVF-first (n=233) | HR (95%CI) | P |
|---|---|---|---|---|
| Outcomes at 5 y | n* | n* | ||
| POCE | 41 (15.4) | 33 (17.4) | 0.93 (0.59-1.48) | .77 |
| All-caused death | 15 (11.2) | 9 (8.9) | 1.36 (0.59-3.10) | .49 |
| Cardiac death | 9 (6.8) | 3 (2.9) | 2.41 (0.65-8.92) | .19 |
| Myocardial infarction | 12 (4.5) | 11 (5.6) | 0.70 (0.34-1.44) | .34 |
| TV myocardial infarction | 5 (1.8) | 4 (2.0) | 0.96 (0.26-3.56) | .95 |
| Any revascularization | 27 (9.7) | 24 (11.7) | 0.86 (0.50-1.49) | .59 |
| TV revascularization | 18 (6.5) | 14 (7.0) | 0.99 (0.49-1.98) | .97 |
| TL revascularization | 15 (5.4) | 10 (4.8) | 1.15 (0.52-2.57) | .73 |
| ST | 6 (2.4) | 5 (2.4) | 0.88 (0.27-2.90) | .84 |
| Outcomes from 5-10 y | ||||
| POCE | 9 (7.5) | 16 (26.6) | 0.28 (0.12-0.62) | .002 |
| All-caused death | 9 (11.6) | 18 (30.6) | 0.38 (0.17-0.84) | .02 |
| Cardiac death | 2 (2.0) | 8 (13.2) | 0.70 (0.52-0.96) | .03 |
| Myocardial infarction | 3 (2.3) | 4 (4.0) | 0.43 (0.10-1.95) | .28 |
| TV myocardial infarction | 1 (0.6) | 2 (2.0) | 0.31 (0.03-3.37) | .33 |
| Any revascularization | 0 | 2 (2.3) | - | - |
| TV revascularization | 0 | 2 (2.1) | - | - |
| TL revascularization | 0 | 1 (0.9) | - | - |
| ST | 1 (0.5) | 4 (11.2) | 0.12 (0.01-1.11) | .06 |
95%CI, 95% confidence interval; HR, hazard ratio; POCE, patient-oriented composite endpoint; ST, stent thrombosis; TL, target lesion; TV, target vessel; TVF, target vessel failure.
Data are expressed as No. (%).
Our study reveals the following main findings: a) Approximately one-third of primary percutaneous coronary intervention STEMI patients experienced at least 1 POCE event during the 10-year follow-up. These patients were characterized by a worse risk profile, including older age, a higher prevalence of diabetes, and more extensive atherosclerotic disease. The remaining patients remained event-free for 10 years. b) The type of the first event (TVF or non-TVF) was not associated with a different risk of subsequent POCE (figure 5). c) However, non-TVF-first patients exhibited an increased risk of very late POCE (5-10 years), mainly driven by a higher mortality rate.
STEMI patients represent a widely investigated population, with several studies reporting the incidence and predictors of adverse events during short- or mid-term follow-up.4,16–19 However, there is a scarcity of data on very long-term outcomes in these patients and most studies have focused only on mortality.5 In our post-hoc analysis of the EXAMINATION-EXTEND trial, we provide unique results by evaluating predictors of POCE over a 10-year follow-up. Our findings reveal that only one-third of STEMI patients experienced a POCE event after 10 years, while the remaining patients remained event-free during this period. Coughlan et al.20 reported results on the 10-year occurrence of POCE in patients treated with DES for acute coronary syndrome, including unstable angina and non–ST-segment elevation myocardial infarction patients. In their analysis, more than 60% of patients had a POCE at 10 years. The discrepancy between these findings and our own can be attributed, at least in part, to the differences in clinical presentation and consequently in the patients’ baseline risk profile, with our STEMI population being younger and with a lower proportion of comorbidities.
Patients experiencing a POCE were characterized by an unfavorable cardiovascular risk profile, including advanced age, diabetes mellitus, previous myocardial infarction, peripheral artery disease, and multivessel coronary disease. Some of these factors, such as age, diabetes mellitus, and multivessel disease, have been widely recognized as predictors of short-term outcomes after STEMI and are included in most of the existing prognostic risk scores.21–25 Our study confirms that these characteristics continue to correlate with the occurrence of adverse events over long-term follow-up. Therefore, their presence should prompt patients and physicians to adopt a more vigilant approach to secondary prevention, even a decade after the index event.
If we take an alternative perspective on our results, it is noteworthy that a high proportion of patients (969 out of 1498, 64.7%) experienced no events during the 10-year period after STEMI. This result is reassuring and suggests that the outcome may be even more favorable in contemporary practice due to advancements procedural aspects and medical treatment over the last decade: since the enrolment of patients in the EXAMINATION trial (from December 31, 2008 to May 15, 2010) numerous important recommendations have been established and implemented in routine clinical practice. These recommendations include the adoption of radial access as the standard approach, use of second-generation DES over bare-metal stents, and the use of potent P2Y12-inhibitors fur dual antiplatelet therapy when not contraindicated.26
Nature of the first event in determining subsequent outcomeIn our analysis, TVF was the first event during follow-up in most patients (56% vs 44%), with a median time to occurrence of 718 [70-1961] days, compared with 1518 [213-2495] days for non-TVF events. These results confirm the previously reported trend of early occurrence of TV vs non-TV-related adverse events,27,28 due to the time frame of the healing process following stent implantation. In contrast, non-TV-related events may be partially attributed to disease progression that involves the entire coronary tree. As a result, these events tend to occur with a more uniform distribution over the years, becoming the most prevalent events during long-term follow-up.
We also investigated whether a TV-related event occurring as the first event would have a significantly different impact on the risk of subsequent outcomes. We hypothesized that patients who experienced an event in the culprit vessel as their first event would be at higher risk of subsequent events than patients experiencing a non-TV-related event. However, our findings indicate that the type of first event does not influence the occurrence of subsequent POCE.
Previous limited data have suggested that early reinfarctions may be associated with an increased risk of subsequent mortality. However, the data were drawn from studies conducted in the thrombolysis era or during the early adoption of primary percutaneous coronary intervention.29–32 Two more recent subanalyses of the HORIZON-AMI trial demonstrated that reinfarction was associated with subsequent cardiac and all-cause mortality,7 while target vessel revascularization increased the risk of subsequent myocardial infarctions without a significant impact on mortality.6
The additional value provided by our analysis is that the risk of recurrent adverse events is not influenced by the type of first event, whether TV or non-TV-related). Therefore, both who experiencing a TVF and those experiencing a non-TVF after STEMI should be followed up with careful secondary prevention.
Of note, the cumulative incidence curves of POCE for TVF-first and non–TVF-first events crossed and diverged during the later follow-up period, suggesting a change in hazards over time. By examining this trend with a landmark analysis, we observed a higher risk of late (5 to 10 years) POCE in the non–TVF-first group, primarily driven by a higher rate of late deaths. A non-TVF event may indicate the progression of atherosclerotic disease throughout the coronary tree, which could identify a population at even higher risk.
Furthermore, it is important to consider that risk factors, comorbidities and drug treatments can change over a 10-year follow-up period. Similarly, the patients’ risk profile, which was initially stratified at the time of enrolment, can evolve over time. These factors may partly explain the delayed shift in hazards between patients with TVF-first and non–TVF-first events. This finding should be considered only as hypothesis-generating and require confirmation by further studies with the same length of follow-up.
LimitationsThis study has several limitations. First, this was a post-hoc analysis and therefore its conclusions can only be considered as hypothesis-generating. Second, the need to use nonproportional hazard models to compare outcomes led to a loss of statistical power. Finally, several clinical and procedural characteristics (eg, Syntax scores, medical treatment during follow-up, etc), as well as other key clinical endpoints, such as stroke, bleeding, vascular complications, and hospitalization for heart failure, were not available for the analysis.
CONCLUSIONSOur post-hoc analysis found that STEMI patients who experienced an adverse event during the 10-year follow-up were characterized by a worse cardiovascular risk profile, but outcomes did not seem to be associated with whether the first event was related to the target vessel of the primary percutaneous coronary intervention or not.
- -
Data on very long-term outcomes in STEMI patients are limited.
- -
It is currently unclear whether the type of first event, and particularly its correlation with the target vessel of the primary percutaneous coronary intervention, influences subsequent outcomes in these patients.
- -
Approximately one-third of STEMI patients experienced a POCE during the 10-year follow-up.
- -
These patients were characterized by a worse cardiovascular risk profile.
- -
Careful secondary prevention is needed regardless of the type of first adverse event occurring during follow-up.
- -
This analysis showed that outcomes did not seem to be influenced by whether the first adverse event was related to the target vessel or not.
The EXAMINATION trial was partially funded by an unrestricted grant from Abbott Vascular to the Spanish Heart Foundation (promoter) during the first 5 years of follow-up. The EXAMINATION-EXTEND study was funded by an unrestricted grant from Abbott Vascular to the Spanish Heart Foundation (promoter).
AUTHORS’ CONTRIBUTIONS. Brugaletta and F.M. Verardi participated in study conception and design. J. Gomez-Lara, V. Jiménez-Diaz, M. Jiménez, P. Jiménez-Quevedo, R. Diletti, P. Bordes, G. Campo, A. Silvestro, J. Maristany, X. Flores, A. De Miguel-Castro, A. Iñiguez, A. Ielasi, M. Tespili, M. Lenzen, N. Gonzalo, M. Tebaldi, S. Biscaglia, P. Vidal-Cales, L. Ortega-Paz, R. Romaguera, J.A. Gomez-Hospital, P.W. Serruys, M. Sabaté and S. Brugaletta contributed to data acquisition. F.M. Verardi performed the analysis and interpretation of data, aided by S. Brugaletta, K. Bujak and P. Tolomeo. F.M. Verardi and S. Brugaletta drafted the manuscript. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published.
CONFLICTS OF INTERESTS. Brugaletta is a consultant for Boston Scientific and iVascular. M. Sabaté has receiving consulting and speaker fees from Abbott Vascular and iVascular. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.