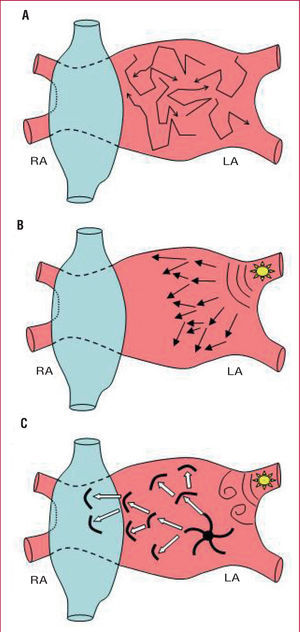
Atrial fibrillation (AF) is the most common arrhythmia seen in clinical practice and is associated with a notable increase in morbidity and mortality. Although there have been considerable advances in the treatment of AF, the results of drug treatment and ablation remain suboptimal.1 This is largely due to the fact that there is still little understanding of the mechanisms responsible for the initiation and maintenance of the arrhythmia. During the past 50 years, the multiple wavelet theory was the sominant concept proposed to explainet AF. According to that hypothesis, AF would be a consequence of functional reentry of multiple wavefronts that would spread throughout the excitable tissue of the atrium, tissue that can become depolarized at the moment it is reached by an activation front (Figure 1A). This hypothesis was questioned by Haissaguerre et al3 who found that extrasystoles and rapid atrial rhythm arising from within the pulmonary veins could initiate and, in some cases, maintain episodes of AF.4,5 According to this focal hypothesis, AF would not only be initiated but also maintained by a very high frequency source arising in or around the pulmonary veins with activation fronts that fractionate and become disorganized in the surrounding tissue, giving rise to what is known as fibrillatory conduction (Figure 1B). Finally, according to the last hypothesis, the beats arising in the pulmonary veins would only act as a trigger for the formation of a functional reentrant circuit generated by abnormal propagation of the electrical impulse in a more extensive area of the atrium (Figure 1C). Using sophisticated optical mapping techniques, Jalife and coworkers6-8 showed that in an experimental sheep model AF is maintained by a "rotor" or vortex anchored in the junction the pulmonary veins and the posterior wall of the left atrium. These rotors occur as a result of a functional microreentrant circuit that causes local high-frequency activation of the tissue, giving rise to fibrillatory conduction in the rest of the atrial tissue.8
Figure 1. Mechanisms underlying maintenance of atrial fibrillation. Current hypotheses. A) Multiple reentrant circuits. Atrial fibrillation would be generated by various wavefronts that would spread throughout the excitable (nonrefractory) atrial tissue. B) Focal hypothesis. Atrial fibrillation would be maintained by continuous discharge of a very high frequency focal source in or around the pulmonary veins. The activation fronts arising from the focus become fractionated and disorganized in the surrounding tissue and give rise to fibrillatory conduction. C) Rotor hypothesis. Atrial fibrillation is triggered by a burst of ectopic beats originating in the pulmonary veins whose wavefronts fragment on arrival at the curvature of the venoatrial junction, generating 2 vortices that rotate in opposite directions. Finally, one of the vortices would become established in the posterior wall, giving rise to the formation of a functional reentry or rotor that would act as rotor that would actas the engine that activates at a high frequency the local tissue generating wavefronts that would fragment and propagate in highly reproducible directions.7 RA indicates right atrium; LA, left atrium.
Focal Versus Reentrant Mechanisms
The thoracic veins, and especially the pulmonary veins, contain myocytes capable of generating spontaneous electrical impulses.3-5 These extrasystolic beats can be generated through various mechanisms related to the formation of abnormal impulses, both as a consequence of increased automaticity and activity triggered by early or delayed afterdepolarizations (Figure 2). However, with the exception of some cases, the role of these extrasystoles or rapid rhythms is only to initiate episodes of AF.9 Elimination of the focus does not achieve such satisfactory results since extrasystoles from other sites could still trigger the arrhythmia.10 Furthermore, analysis of the results of ablation has shown that isolation of successive veins gives rise to a gradual increase in the cycle length of AF that ultimately stops it in a large number of cases.11 In addition, termination of AF during ablation of the ostium of a pulmonary vein often occurs before complete isolation of the vein is achieved, an observation that is more consistent with the elimination of the substrate necessary for a reentrant mechanism than with the elimination of foci responsible for AF initiation/maintenance.11
Figure 2. Cellular mechanisms responsible for the formation of cardiac arrhythmias. A) Representative example of a transmembrane action potential arising from electrical activation of an atrial cardiomyocyte; the phases (0-4) are determined by a series of inward and outward ion currents. The duration of the action potential and, therefore, the refractory period, essentially depend on the equilibrium between the inward and outward ion currents during phases 2 and 3.16 B) Reentry occurs as a consequence of sequential activation of 2 contiguous regions of tissue (α and β) that possess different refractory periods and/or conduction velocities. C) Automaticity occurs as a consequence of the increase in the rate of discharge (phase 4) of cells able to depolarize spontaneously. D) The triggered activity is a consequence of the appearance of afterdepolarizations that reach the depolarization threshold and generate an action potential. DT indicates depolarization threshold; RP, refractory period; AD, afterdepolarization.
Refractory Period of the Left Atrium and Inducibility of Atrial Fibrillation
Reentrant circuits can exist as a single circuit giving rise to a regular discharge (Figure 1C) or as multiple coexisting circuits (Figure 1A) producing irregular fibrillatory activity. In both cases, reentry occurs as a consequence of abnormal propagation of the electrical impulse between 2 areas of tissue. Following the depolarization that initiates the action potential, Na+ channels are inactivated and cannot open again until the cell is repolarized to a potential of approximately 60 mV, a period of time known as the refractory period (Figure 2A). The equilibrium between the inward and outward ion currents during the plateau phase of the transmembrane action potential determines its duration and, therefore, the refractory period of the tissue and the possibility or ease of maintaining reentry. Thus, the main determinant of the maximum excitation frequency of a region of the myocardium corresponds to the refractory period of the tissue, such that longer refractory periods make it more likely that a circulating impulse encounters tissue that is still refractory and terminates reentry.12
In this issue of Revista Española de Cardiología, Fernández-Lozano et al13 provide data supporting reentry as the principal mechanism for maintenance of AF, the substrate for which would be located in the posterior wall and pulmonary veins. Using an in vivo experimental model in pig hearts with epicardial mapping, programmed stimulation of the right and left atria was performed with perfusion of methacholine at increasing doses in an effort to analyze the relationship between the refractory period in different regions of the atrium and inducibility of AF. The authors observed a tendency towards greater inducibility of AF when programmed stimulation was performed from the left atrium; the refractory periods of the pulmonary veins and the posterior wall were the main determinants. Thus, there was a higher probability of inducing AF in those animals with shorter refractory periods, especially in the posterior wall and the pulmonary veins. However, with the exception of the lateral wall of the right atrium, no correlation was observed between the cycle length of AF and the refractory periods of the different points analyzed.
Electrophysiology of the Pulmonary Veins
Electrophysiologic analysis of the pulmonary veins has revealed the existence of all the ingredients necessary to sustain reentry. The myocytes of the pulmonary veins display transmembrane action potentials with a slower upstroke velocity in phase 0 and a shorter duration, which is translated into a shortening of the refractory period and a slowing of the conduction velocity.14 In addition, sudden changes in the arrangement of the muscle fibers and their orientation in the posterior wall give rise to the phenomena of slowing and discontinuous conduction.15 Experimental studies using optical mapping techniques have shown that AF is maintained by functional microreentrant circuits usually located in the posterior wall of the left atrium and the venoatrial junction.6-8,14 The preference of these rotors for the left atrium and especially for the regions close to the pulmonary veins, appears to be due to differences in the distribution of the ion channels that make the refractory periods in that zone particularly short, thereby favoring reentry.16
Various studies performed in humans have analyzed the electrophysiologic characteristics of the pulmonary veins and the venoatrial junction, along with other atrial regions, although none have assessed the posterior wall in isolation. The results of those studies demonstrate that 1) the distal portion of the pulmonary veins has a shorter refractory period than the proximal portion, the venoatrial junction, or the left atrial appendix5,15,17; 2) AF is more likely to be induced through stimulation of the distal portion of the pulmonary veins17; 3) the patients in whom AF is induced show significantly shorter refractory periods in the proximal portion of the pulmonary veins than in other regions of the left atrium17; and 4) the venoatrial junction presents properties of discontinuous conduction and dispersion of repolarization, LEPOS to conduction block in the vicinity of areas of slow conduction and the formation of reentrant circuits.14,17 Thus, the posterior wall and the pulmonary veins constitute the ideal substrate to facilitate the existence and maintenance of reentry. However, that does not prove that reentry is the principal mechanism of AF maintenance. In a recent study, we showed that perfusion of adenosine, which inhibits automaticity and triggered activity and shortens the refractory period, increased the rate of local activation in the atrial tissue (dominant frequency) during AF, a phenomenon that is only possible if AF is maintained through a reentry mechanism.18 Thus, reentry is also the principal mechanism for the maintenance of AF in humans.
Towards a Unified Hypothesis?
Where is the substrate necessary for the maintenance of atrial fibrillation? In the pulmonary veins or the posterior wall? The results of catheter ablation demonstrate that both structures are important. The outcome of ablation has improved through the use of procedures that do not involve the interior of the veins but rather the venoatrial junction or the antrum; this approach, primarily performed to avoid stenosis of the pulmonary vein, has also been demonstrated to be more effective than purely focal ablation.10 However, segmental ablation, that aims to simply Achieve the electrical disconnention of the veins, also yields poorer results than circumferential isolation, which generates a complete ring of lesions adjacent to the ostium of the veins and includes part of the posterior wall and the venoatrial junction within the isolated zone.19 On the other hand, although up to a third of the isolated veins exhibit dissociated rhythms, only 5% present tachycardias.20 The low incidence of tachycardias in the pulmonary veins following their isolation underlines the key role played by the venoatrial junction and adjacent regions (posterior wall) in the maintenance of AF. The strength of the study by Fernández-Lozano et al13 lies in its support for the key role played by the posterior wall of the atrium as a substrate for the maintenance of AF.
The study has a series of limitations inherent to the experimental methods used: 1) limited mapping density (a maximum of 24 bipolar signals in total), which limits the spatial resolution of the findings (eg, proximal vs distal in the pulmonary veins); 2) inducibility was not analyzed for each of the areas in which the refractory period was measured, but rather, only in the right atrium and the left posterolateral atrium; 3) no analysis was performed of activation maps or conduction times during AF, studies that could have provided useful additional information; and 4) the absence of a correlation between the refractory period and the cycle length of AF in the posterior wall that is difficult to explain beyond the methodological differences and contradicts the findings of other studies.12
Unlike in a large number of other arrhythmias, AF continues to represent a therapeutic challenge that has stimulated the search for new treatment modalities. The mechanisms underlying initiation and maintenance of AF are still not known with any accuracy and generate intense debate. A better understanding of those mechanisms will allow safer and more effective treatment strategies to be developed based on the pathophysiology of the disease. Experimental animal models continue to represent a valuable tool for the study of AF. While the study by Fernández-Lozano et al13 represents a valuable contribution to the analysis of the mechanisms responsible for the maintenance of AF, the search must continue.
ACKNOWLEDGMENTS
I am grateful to Drs José Jalife and Jesús Almendral for critical review of this article and to the Spanish Society of Cardiology (Sociedad Española de Cardiología) for financial support (Basic and Clinical Research grants, 2005).
Correspondence: Dr. F. Atienza Fernández.
Servicio de Cardiología. Hospital General Universitario Gregorio Marañón.
Dr. Esquerdo, 46. 28007 Madrid. España.
E-mail: fatienzaf@secardiología.es