Keywords
INTRODUCTION
Low cardiac output syndrome (LCOS) is one of the most serious post-operative outcomes of cardiac surgery. It is associated with high morbi-mortality, increased use of resources, and a prolonged stay in a critical care unit.1,2
The incidence of LCOS ranges between 3% and 20% in different studies, depending on factors such as the age of the population studied, the degree of pre-operative ventricular dysfunction, and the type of intervention. Treatment of LCOS usually includes the use of inotropic beta-adrenergic receptor agonists and/or phosphodiesterase III inhibitors, vasodilatory agents and, on occasions, intra-aortic balloon counterpulsation.3-5
Levosimendan (LS) is a new inotropic drug which belongs to the group of drugs known as calcium sensitizers. These act on the cardiovascular system through a double (at least) mechanism; firstly, through their interaction with the contractile proteins and remodeling which leads to enhancement of myocardial contractility and, secondly, through activation of the adenosine triphosphate-sensitive potassium channel (KATP). The end result includes arterial and venous vasodilation.6,7
The action of LS has been widely studied in populations with complicated acute myocardial infarction and decompensated heart failure. Some of these studies have shown that the drug can reduce mortality. Less is known about its effects in the treatment of post-operative LCOS after cardiac surgery.8-10
The primary objective of the present study was to compare hospital mortality in patients with postoperative LCOS after coronary surgery with extracorporeal circulation (ECC) treated with LS or dobutamine. Secondary objectives were to assess the incidence of postoperative complications (morbidity), the need for additional inotropic agents or vasopressors, need for balloon counterpulsation, and time spent in intensive care.
METHODS
Population
Patients receiving coronary surgery with ECC in either of 2 teaching hospitals between December 1, 2003 and December 1, 2006 were included consecutively and prospectively in the study.
Diagnosis of LCOS was established from hemodynamic data obtained using a Swan-Ganz catheter placed systematically during surgery after anesthetic induction. For the purposes of the present study, LCOS was defined as: pulmonary capillary pressure 316 mm Hg, cardiac index <2.2 L/min/m2, and mixed venous saturation <60%. Diagnosis of LCOS was performed in the first 6 hours after the intervention and after excluding or correcting temperature anomalies (hypothermia), pre-charge (hypovolemia), and the cardiac rhythm (bradycardia), cardiac tamponade, or postoperative ischemia. Patients meeting LCOS criteria were randomized using a computer generated algorithm to receive either LS at a loading dose of 10 µg/kg for 1 h, followed by 0.1 µg/kg/min for 24 h, or dobutamine 5 µg/kg/min (if a favorable hemodynamic response was not observed, the dose was increased initially to 7.5, then to 10 and finally to 12.5 µg/kg/min successively, at 15 min intervals). Dobutamine was chosen as it is the standard treatment for postoperative low output in the participating hospitals (and in Argentina in general) because of its hemodynamic effects, which are comparable to milrinone, but at a lower cost.
In cases where low cardiac output persisted, a second inotropic drug was added to the treatment regime (milrinone at a dose of 0.375 µg/kg/min). If LCOS remained unresolved, adrenalin was added as a third-line treatment at a dose of 1-10 µg/min. Finally, circulatory support was provided through balloon counterpulsation if required.11
LS was administered via bolus in order to achieve a rapid hemodynamic effect. Administration was every 60 min (instead of the usual 10 min) after earlier experiences in our unit which showed it was possible to combine a rapid onset of action without producing the hypotension which frequently accompanies "conventional" administration. The maintenance dose chosen was within the usual range (0.05-0.2 µg/kg/min) and, again, it was chosen after a series of prior successful experiences both in a postoperative and preoperative context in patients with severe left ventricular dysfunction.12
Hemodynamic analyses were performed on diagnosis of LCOS (baseline, or time 0) and at time 1, or 1 hour after treatment initiation, and times 2, 3, 4, and 5 (6, 12, 24, and 48 h after diagnosis, respectively). Anesthetic and surgical techniques were similar. Fentanyl (10-20 µg/kg) was used with midazolam (0.03-0.05 mg/kg) to induce anesthesia. Intubation was facilitated using pancuronium. Sevofluorane, fentanyl, and propofol infusion (1-4 mg/kg/h) were employed as maintenance treatment. All procedures were performed using ECC and general myocardial ischemia through aortic clamping and electromechanical arrest with a cold, hyperpotasemic, blood cardioplegic solution administered intermittently.
Exclusion Criteria
Patients were excluded if they received emergency surgery, valvular, or combined techniques, surgery without ECC, low use of preoperative balloon counterpulsation or inotropic drugs, or if they had preoperative kidney failure (defined as glomerular filtration rate <59 mL/min).
Postoperative Complications (Morbidity)
The following complications were defined:
- Perioperative infarction: new pathologic Q waves (>0.04 s) in 2 contiguous derivations with an increase in creatine kinase (CK) >1000 U and MB fraction >10%
- Vasoplegia: arterial hypotension associated with reduced peripheral resistance of <800 din/s/cm5, with low filling pressures, normal or raised cardiac index, and need for vasopressors
- Kidney failure: increase in creatine of >50% associated or not with oliguria (diuresis <0.5 mL/kg/h)
- Prolonged ventilatory assistance: length of time on ventilator 324 h
- Stroke: a new focal neurological deficit or coma lasting over 48 h, after rejecting metabolic causes
- Systemic inflammatory response syndrome: presence of 2 or more of the following13: temperature >38 or <36oC; cardiac frequency >90 beats/min; respiratory frequency >20/min or PCO2 <32 mm Hg; leukocytosis >12 000 or leukocytopenia <4000, or >10% of immature forms
- Sepsis: inflammatory response syndrome together with evidence of infection
- Pneumopathy: new pulmonary consolidation accompanied by a compatible clinical profile (fever, purulent expectoration) or documented infection site
- Adult respiratory distress: hypoxemia (ratio PaO2/FiO2 <150) which does not resolve with use of positive pressure or PEEP with a reduction in pulmonary distensibility and diffuse infiltrate
- Hospital mortality: death during hospital stay or in the first 30 days after the intervention
The following criteria were established for suspension of treatment: severe arterial hypotension, systolic blood pressure of <80 mm Hg with no response to volume expansion (2 charges of up to 500 mL each) or use of vasopressor drugs (phenylephrine up to 50 µg/min, noradrenalin up to 10 µg/min, vasopressin up to 0.04 U/min), development of myocardial ischemia (angina or elevated ST segment ST >2 mm), supraventricular tachycardia with a cardiac frequency of >130 beats/min, hemodynamic decompensation or ischemia, or sustained ventricular tachycardia without electrolytic alterations.
The study followed the principles of the Helsinki protocol and was approved by an institutional review board. Informed consent was obtained from the patient or a relative.
Statistical Analysis
Between group differences were analyzed using c2 or Fisher's exact test for categorical variables. The Student t test was used for continuous variables. Odds ratios and their corresponding 95% confidence intervals (CI) were used to analyze relationships between discrete variables. Numeric variables were expressed as means (standard deviation). Length of hospital stay was analyzed by comparing medians using the Mann-Whitney test. A P value less than .05 was considered significant.
RESULTS
Over the study period, a total of 1004 heart surgeries were performed and 137 patients (13.6%) met the criteria for postoperative LCOS. After informed consent was obtained from the patient or relative, 69 patients were randomized to treatment with LS and the remaining 68 patients were treated with dobutamine. Diagnosis of LCOS was made in all cases within 3 hours of the intervention (2.3 h for patients treated with LS and 2.2 h for those treated with dobutamine). Treatment was initiated approximately 30 min after diagnosis in all patients (21.4 min in the LS group and 19.8 min in the dobutamine group).
The 2 study groups were similar in terms of their overall characteristics, preoperative medication, and the main variables describing the intervention (Tables 1 and 2).
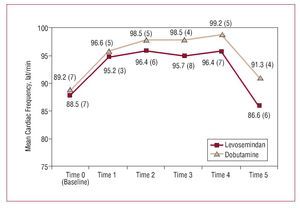
Figures 1-5 show the principle hemodynamic findings. Both agents had beneficial effects on hemodynamic parameters, though the favorable effect was more marked and of faster onset in patients treated with LS.
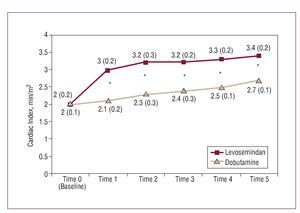
Figure 1. Comparative evolution of cardiac index during treatment. *Between group difference statistically significant (expressed as mean [standard deviation] and calculated using the Student t test).
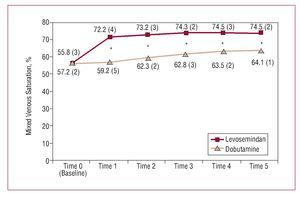
Figure 2. Comparative evolution of mixed venous saturation during treatment. *Between group difference statistically significant (expressed as mean [standard deviation] and calculated using the Student t test).
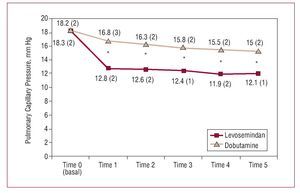
Figure 3. Comparative evolution of pulmonary capillary pressure during treatment. *Between group difference statistically significant (expressed as mean [standard deviation] and calculated using the Student t test).
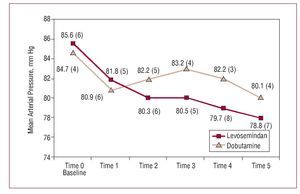
Figure 4. Comparative evolution of arterial blood pressure during treatment. Differences were not statistically significant.
Figure 5. Comparative evolution of cardiac frequency during treatment. Differences were not statistically significant.
The mean dose of dobutamine was 9.2 (8.3) (range, 5-11) µg/kg/min in patients who had not been previously treated with beta-blockers and 10.5 (8-13) µg/kg/min in patients who had received beta-blockers previously.
A total of 23 patients (16.8%) died during the study period, 6 (8.7%) in the LS group and 17 (25%) in the dobutamine group (P=.01; OR=0.29; 95% CI, 0.09-0.85). None of the deaths occurred within 48 h of initiating treatment; the first death occurred 99 h after starting treatment in the LS group and 96 h after initiating treatment in the dobutamine group (the difference was not significant).
Causes of death in the dobutamine group were: cardiac, 10 patients (8 cases of refractory low output and 2 patients with refractory ventricular arrhythmia), neurological in 2 patients, and multi-organ failure in 5. In the LS group, causes of death were: cardiac in 2 patients, neurological in 1 patient, and multi-organ failure in the remaining 3 patients. The difference in cardiac-based mortality was statistically significant (P=.014; OR=0.17; 95% CI, 0.03-0.89); differences for the other causes of death were not statistically significant.
Table 3 shows the principal postoperative complications. There were fewer complications in the LS group. The difference was statistically significant for perioperative infarction, systemic inflammatory response syndrome, vasoplegia, sepsis, kidney failure, and ventricular arrhythmia. Prolonged ventilatory assistance was less frequently required in the LS group. It was not necessary to suspend the treatment protocol in any of the 137 patients treated, though volume expansion was required in 20 LS patients and in 51 patients in the dobutamine group (29% vs 75%; P=.0001; OR=0.25; 95% CI, 0.12-0.55).
Additional inotropic agents were less frequently required in the LS group. A second inotropic agent was required in 6 patients in the LS group (8.7%) compared to 25 (36.8%) patients in the dobutamine group (P<.0001; OR=0.16; 95% CI, 0.05-0.47), while a third inotropic was required in 2 patients (2.9%) in the LS group and in 10 patients (14.7%) in the dobutamine group (P=.014; OR=0.17; 95% CI, 0.03-0.89). Two patients (2.9%) required balloon treatment in the LS group compared to 10 (14.7%) in the dobutamine group (P=.014; OR=0.17; 95% CI, 0.03-0.89).
Eight patients (17.6%) in the LS group required vasopressors compared to 21 (25%) in the dobutamine group (P=.005; OR=0.29; 95% CI, 0.11-0.75).
Mean (median) length of stay in intensive care was 88 h (66-156 h for the first and third quartiles, respectively). Length of stay was lower in the LS group: 66 h (58-74) compared to 158 h (106-182) (P<.05).
The postoperative ejection fraction, which was assessed before discharge from hospital, was 54.23% (4.65%) in the LS group compared to 45.64% (5.21%) in the dobutamine group. A second evaluation 90 days after the intervention gave values of 57.65% (3.37%) in patients treated with LS and 53.46% (4.20%) in patients who received dobutamine.
DISCUSSION
This is one of the first randomized studies to show a decrease in hospital mortality, smaller number of complications, reduced length of stay in critical care units, and smaller use of additional inotropic or vasopressor agents and balloon counterpulsation in patients with postoperative LCOS treated with LS or dobutamine. Although hemodynamic parameters improved with both drugs, the improvement was larger and onset of action was quicker in patients treated with LS. The larger gains with LS can be seen in parameters which evaluate systolic function, such as the cardiac index (50% increase in the first hour of treatment), as well as in variables used to measure tissular metabolic condition, such as mixed venous saturation (which increased 29.6% in the same period). Added to this was the 35.5% decrease in pulmonary capillary pressure values, again within the first hour of treatment.
These observations support our initial expectations. Under experimental conditions, LS has been shown to be associated with greater contraction force in samples of isolated heart tissue, both in comparison to beta-adrenergic receptor agonists as well as to phosphodiesterase III inhibitors. The quicker onset of action has also been noted. A quicker reversal of LCOS, as with any unfavorable hemodynamic profile, is important for prognosis, as has been shown recently with sepsis. The lower number of postoperative complications can be explained by the quicker reversal, which no doubt also played a role in the lower rate of observed mortality.14-17
The specific, and in some cases differential, actions of LS in comparison to other inotropic medicines provide the rest of the interpretation. LS has been shown to have a dual action: it acts first on troponin C, which it sensitizes to calcium, thereby increasing contractility without increasing cyclical adenosine monophosphate (AMPc) nor the intracellular quantity of calcium nor consumption of myocardial oxygen. These effects have been associated in conventional inotropics with cardio-toxicity, progression of heart failure, development of ischemia, proarrhythmic effect, major apoptosis, and increased mortality.18-20
Additionally, LS, through the activation of the KATP, produces vasodilatory effects (arterial, coronary, and venous), decreased pre-load and post-load, and coronary, pulmonary, and mammary vasodilation. The same mechanism of action suggests both anti-ischemic and cardio-protective effects for LS. The agent itself would generate pharmacological preconditioning which favors the recovery of stunned myocardium. This would be particularly beneficial in situations of ischemia-reperfusion which are frequent in postoperative cardiac surgery with ECC, where loss of calcium sensitivity by the contractile apparatus is one of the mechanisms present. LS, in contrast to other inotropic agents, increases reduced sensitivity. Beta-adrenergic receptor agonists, on the other hand, have been shown in experiments to increase that desensitization. Several studies have also indicated a reduction in myocardial damage as shown by smaller increases in troponin. In the present study, we consider the lower incidence of perioperative infarction to likely be an expression of the aforementioned cardio-protective effect.21-24
On the other hand, the fact that increased sensitization is limited to the systole means that LS does not alter the diastolic properties or have a positive lusotropic effect.
Another of the drug's notable characteristics is the reduction in the values of inflammatory parameters, such as cytokines, a fact which has not been evaluated in postoperative patients, although our lower incidence of inflammatory response syndrome and vasoplegia could be evidence of the aforementioned anti-inflammatory effect.18,25
There have been relatively few reports of the use of LS in postoperative LCOS and most have not been from randomized trials. Ploecl, Labriola, and Tokuda have reported on 3 clinical series, though the number of patients included was low (12, 11, and 9 patients, respectively). Nevertheless, the 3 reports coincided in showing a significant improvement in hemodynamic parameters with LS.26-28
Álvarez et al evaluated a total of 41 patients with postoperative LCOS in a randomized study which, like those mentioned above, compared LS and dobutamine on hemodynamic parameters. They concluded that both drugs led to an improvement on those parameters, though LS significantly reduced systemic valvular and pulmonary resistance with a larger decrease in systemic arterial, pulmonary, central venous, and pulmonary capillary pressure.29
In another randomized study, Tasouli et al compared 45 patients with LCOS treated with LS at 2 different time points. The patients had been treated with inotropics and/or balloon counterpulsation. The study showed better results in those who were treated earlier, with a shorter time on inotropic support, a lower incidence of sepsis, and a shorter length of stay in intensive care units and in hospital in general.30
It should be borne in mind that the LS doses used in these studies differed from those used here. Our choice of a lower loading dose administered over a longer period was based on prior experience with the drug which had showed that this approach was associated with fewer adverse events. In particular, we had found that this approach led to a lower incidence of severe arterial hypotension (only 11.6% of patients required vasopressors), and without the need to suspend treatment in any case.
The increase in the postoperative ejection fraction was reasonable and as expected when taking into account that, although it was low in the preoperative phase, the majority of dysfunctions were ischemic in nature (17% of patients with a previous infarction). Likewise, patients in the LS group recovered function more quickly.
Study Limitations
In the first place, although this is one of the largest series of LCOS patients treated with LS followed to date, the numbers are still low. Nevertheless, all of the patients randomized were treated effectively and, in this sense, the low number included could have been beneficial. Second, we did not use a double blind design. A double blind trial would be very useful in helping to confirm the results presented here. The fact that a double blind design was not used could have influenced our results, although the 2 study groups were comparable and those responsible for data collection and analysis were unaware of patient assignation. The dose of LS used could also be questioned, although this tends to vary across studies, as mentioned above.
Despite these considerations, the favorable results nevertheless provide a stimulus for further research on the use of LS in this context. Such research would be useful in confirming the results of the present study.
CONCLUSIONS
In this randomized comparison of dobutamine and LS in patients with postoperative LCOS, LS was associated with greater improvement on hemodynamic parameters and a faster onset of action, as well as with lower mortality and complication rates, lower use of additional inotropic and vasopressor drugs, less need for balloon counterpulsation, and shorter length of stay in intensive care.
These findings suggest that LS is an effective treatment for this particular clinical condition. Studies with larger numbers of patients will help to determine the value of the present findings.
SEE EDITORIAL ON PAGES 454-7
ABBREVIATIONS
CI: confidence interval
ECC: extracorporeal circulation
KATP: adenosine triphosphate-sensitive potassium channel
LCOS: low cardiac output syndrome
LS: levosimendan
Correspondence:
Dr. R. Levin,
1500 21st Avenue South, Nashville, 37212 Tennessee, United States
E-mail: rllevin@gmail.com; Ricardo.levin@vanderbilt.edu
Received October 3, 2007.
Accepted for publication January 22, 2008.