By 2022, 9 centers had been accredited by the Spanish Society of Cardiology for the atrial fibrillation (AF) process. Our objective was to evaluate the performance of these centers based on the quality indicators (QIs) proposed by the European Society of Cardiology (ESC) in 2020.
MethodsAdults with AF who were attended in the cardiology departments of participating centers during the second week of May 2019 were included in a retrospective registry (n=797, age 72±11 years, 60% male). Key ESC QIs were assessed.
ResultsCHA2DS2-VASc, HAS-BLED scores, and serum creatinine levels were documented in 24.9%, 6.1%, and 96.2% of patients, respectively. Anticoagulation was appropriately prescribed in 90.6% of high-risk patients according to the CHA2DS2-VASc score, but was inappropriately prescribed in 57.8% of low-risk patients. Among all patients, 84.1% received high-quality anticoagulation. Inappropriate antiarrhythmic drugs were prescribed in 7.2% of patients with permanent AF, 2.9% of those with structural heart disease, and 0.0% of those with end-stage kidney disease. Catheter ablation was offered to 70% of patients with symptomatic paroxysmal or persistent AF after the failure or intolerance of 1 antiarrhythmic drug. All modifiable risk factors were documented in 59.3% of patients. Rates of all-cause mortality, ischemic stroke or transient ischemic attack, and major bleeding were 8.1, 0.8, and 2.56 per 100 patients/y, respectively. QIs for anticoagulation and outcomes were similar between general cardiology and tertiary referral centers.
ConclusionsAlthough accredited centers in Spain demonstrated good performance in many of the ESC QIs for AF, there remains room for improvement. These data could serve as a starting point for enhancing the quality of care in this population.
Keywords
Atrial fibrillation (AF) is a major public health problem, with a huge and growing burden of morbidity and mortality.1 The 2020 European Society of Cardiology (ESC) guidelines on AF proposed a set of quality indicators (QIs) aimed at improving the quality of AF care,2,3 and granted a Class IIa recommendation to the introduction of tools to measure the quality of care and identify opportunities to improve treatment quality and patient outcomes in AF.2 However, there are limited data on the evaluation of these QI in clinical settings.4–6 In 2016, the Spanish Society of Cardiology (SEC) launched a strategy for quality improvement in cardiovascular disease, known as SEC-CALIDAD (SEC-Quality), which includes several key elements available on the SEC website.7 One of these elements, the RECALCAR registry,8–17 compiles data on discharges from all public Spanish hospitals, with information on resources, personnel, activity, and the structure of each cardiology unit. Another key component of the SEC-CALIDAD strategy is the SEC-EXCELENTE accreditation program. A set of processes and procedures were selected for accreditation based on their priority, as determined by the Quality Committee of the SEC; among these were heart failure18,19 and AF processes. This voluntary accreditation process was offered to all cardiology units in Spain by the SEC, which also encouraged them to apply. By 2022, 9 centers had been granted SEC-EXCELENTE accreditation.
Our main objective was to evaluate the main QIs proposed by the ESC in 2020 in these centers. A secondary objective was to identify possible associations of these QI with the resources and structure of participating centers.
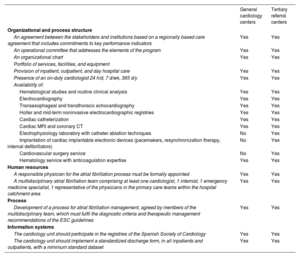
METHODSThe SEC-EXCELENTE in AF accreditation processSince 2017, all interested centers have been required to submit a formal application to the SEC, including documentation proving compliance with the minimum requirements for the initial SEC-EXCELENTE accreditation in AF, which are available on the SEC website7 and detailed in table 1. Once compliance with these quality standards is verified, the unit receives an initial accreditation. A reaccreditation is planned 5 years after the initial accreditation, based on participation in a registry and an evaluation of results.
Characteristics and required standards of the centers with the SEC-EXCELENTE in atrial fibrillation accreditation in Spain
| General cardiology centers | Tertiary referral centers | |
|---|---|---|
| Organizational and process structure | ||
| An agreement between the stakeholders and institutions based on a regionally based care agreement that includes commitments to key performance indicators | Yes | Yes |
| An operational committee that addresses the elements of the program | Yes | Yes |
| An organizational chart | Yes | Yes |
| Portfolio of services, facilities, and equipment | ||
| Provision of inpatient, outpatient, and day hospital care | Yes | Yes |
| Presence of an on-duty cardiologist 24 h/d, 7 d/wk, 365 d/y | Yes | Yes |
| Availability of: | ||
| Hematological studies and routine clinical analysis | Yes | Yes |
| Electrocardiography | Yes | Yes |
| Transesophageal and transthoracic echocardiography | Yes | Yes |
| Holter and mid-term noninvasive electrocardiographic registries | Yes | Yes |
| Cardiac catheterization | Yes | Yes |
| Cardiac MRI and coronary CT | Yes | Yes |
| Electrophysiology laboratory with catheter ablation techniques | No | Yes |
| Implantation of cardiac implantable electronic devices (pacemakers, resynchronization therapy, internal defibrillators) | No | Yes |
| Cardiovascular surgery service | No | Yes |
| Hematology service with anticoagulation expertise | Yes | Yes |
| Human resources | ||
| A responsible physician for the atrial fibrillation process must be formally appointed | Yes | Yes |
| A multidisciplinary atrial fibrillation team comprising at least one cardiologist, 1 internist, 1 emergency medicine specialist, 1 representative of the physicians in the primary care teams within the hospital catchment area | Yes | Yes |
| Process | ||
| Development of a process for atrial fibrillation management, agreed by members of the multidisciplinary team, which must fulfil the diagnostic criteria and therapeutic management recommendations of the ESC guidelines | Yes | Yes |
| Information systems | ||
| The cardiology unit should participate in the registries of the Spanish Society of Cardiology | Yes | Yes |
| The cardiology unit should implement a standardized discharge form, in all inpatients and outpatients, with a minimum standard dataset | Yes | Yes |
By 2022, all units with SEC-EXCELENTE in AF accreditation participated in a retrospective, observational, noninterventional registry with single access to patients’ clinical histories. This registry was planned in 2 phases. In the first phase, the objective was to evaluate the QI defined in the SEC-EXCELENTE in AF process. Based on these data, a comprehensive report—containing both global and detailed results for each center—was sent to the person responsible for the AF process and the head of department at each center. They were tasked with evaluating the results, sharing the data with health care professionals, and designing and implementing quality improvement measures. A second phase of data collection is planned for the future to measure changes in QIs. This study describes the evaluation of the main ESC QIs in AF during the first phase of the registry.
Inclusion criteriaAll adults (age ≥ 18 years) with a diagnosis of AF, whether previously established or made during medical attention from May 6 to 12, 2019, in the participating cardiology units (both outpatient clinics and hospitalization wards), were included in the registry, with no exclusion criteria. At that time, most centers had committed to managing patients with AF according to SEC-EXCELENTE standards, and a 7-day period was considered sufficient to balance feasibility and representativeness. Data from the Minimum Basic Dataset of hospital discharges were not suitable for this purpose, as outpatients are not yet included in this database. The date of inclusion was based on either the outpatient clinic visit or the date of hospital discharge.
Study procedures and ethical issuesThere was only 1 study procedure, which involved accessing patients’ clinical records to collect baseline and follow-up variables, followed by an anonymization process to permanently dissociate personal data from the clinical information included in the database. The principal investigator at each center (the person responsible for the AF process), in agreement with the head of department, appointed clinicians with care responsibilities to collect and anonymize the data. These clinicians were either clinical cardiologists or cardiology residents, working under the supervision of the principal investigators, who were electrophysiologists in 4 tertiary centers and clinical cardiologists in the remaining units. All variables were entered into an online database platform provided by the SEC, which included tools to ensure data validity and integrity. The study protocol was approved by the Research Ethics Committee of each center and complied with the recommendations of the Declaration of Helsinki for medical research. Since the study data were purely clinical-care, anonymous, and dissociated from personal information, the Research Ethics Committees determined that informed consent was not necessary.
Study variablesDemographic and clinical data were collected, with a focus on risk factors, established heart disease, comorbidities, thrombotic and bleeding scores, AF characterization, procedures, physical examinations, complementary tests, antithrombotic management, and concomitant treatment. The presence of CHA2DS2-VASc and HAS-BLED scores in medical records was noted, and these scores were independently calculated using available variables. All patients were followed up until December 31, 2022, with records of stroke, transient ischemic attack, major bleeding (as defined by the International Society on Thrombosis and Haemostasis)20 and all-cause death. The main QIs were calculated according to ESC definitions.3 Data on the structure and resources of centers were obtained from the 2019 RECALCAR survey, categorizing centers into 2 types: those with arrhythmia units offering AF ablation programs and cardiac surgery facilities (tertiary referral hospitals) and those without (basic cardiology hospitals). All centers had a cardiology department with an outpatient clinic, hospitalization wards, and echocardiography and catheterization laboratories. Baseline data and QIs were compared between the 2 types of centers.
Statistical analysisQuantitative variables were tested for normality and are expressed as mean±standard deviation or median (25th-75th percentile), as appropriate. Qualitative variables are reported as numbers and percentages, with 95% confidence intervals provided for QIs. Event incidences are described as rates per 100-patients/y. The Student t test for independent data, Mann Whitney U test and chi-square test were used, as appropriate, to compare subgroups of variables. For QI comparisons, odds ratios and confidence intervals were calculated. Crude values were adjusted for variables with an imbalance between groups with a P value <.1 (table 2) using multivariable logistic regression models, with general cardiology centers as the reference group. For mortality, stroke or transient ischemic attack and major bleeding rates, hazard ratios and confidence intervals were calculated, and adjusted similarly using multivariate Cox proportional hazards models. SPSS software version 25.0 (IBM Corporation, United States) was used and P<.05 values were considered statistically significant.
Baseline features of the patients according to the type of inclusion center
| Variable | All series | General cardiology centers | Tertiary referral centers | P |
|---|---|---|---|---|
| Number of patients | 797 | 175 | 622 | |
| Demographics | ||||
| Age, y | 72±12 | 73±10 | 71±6 | .105 |
| Age>75 | 346 (43.5) | 79 (45.7) | 267 (42.9) | .52 |
| Sex (male) | 476 (59.7) | 112 (64.0) | 364 (58.5) | .192 |
| Hospitalized at inclusion | 180 (22.6) | 17 (9.7) | 163 (26.2) | <.000 |
| Risk factors | ||||
| Hypertension | 548 (68.8) | 126 (72.0) | 422 (67.8) | .295 |
| Diabetes | 211 (26.5) | 47 (26.9) | 164 (26.4) | .897 |
| Smoker (last year) | 58 (7.4) | 13 (7.8) | 45 (7.3) | .834 |
| Hypercholesterolemia | 422 (54.9) | 85 (55.6) | 337 (54.7) | .850 |
| Excessive alcohol intake | 33 (4.3) | 8 (5.1) | 25 (4.1) | .574 |
| Obesity | 193 (37.3) | 23 (30.3) | 170 (38.5) | .172 |
| Sedentary lifestyle | 598 (78.1) | 98 (65.3) | 500 (81.2) | <.000 |
| Cardiological history | ||||
| Previous heart disease | 487 (61.1) | 114 (65.1) | 373 (60.0) | .215 |
| Previous admission for heart failure | 194 (24.3) | 45 (25.7) | 149 (24.0) | .632 |
| Ischemic heart disease | 190 (23.8) | 35 (20.0) | 155 (24.9) | .177 |
| Percutaneous revascularization (stents) | 122 (15.3) | 23 (13.1) | 99 (15.9) | .368 |
| Coronary surgery | 44 (5.5) | 5 (2.9) | 39 (6.3) | .081 |
| At least moderate valvular heart disease | 277 (34.8) | 56 (32.0) | 221 (35.5) | .386 |
| Mitral stenosis (moderate-severe) or mechanical valve prosthesis | 51 (6.4) | 6 (3.4) | 45 (7.2) | .069 |
| Cardiomyopathy | 59 (7.4) | 13 (7.4) | 46 (7.4) | .988 |
| Other heart disease | 70 (8.8) | 22 (12.6) | 48 (7.7) | .045 |
| Comorbidities | ||||
| Renal failure | 260 (32.6) | 49 (28.0) | 211 (33.9) | .140 |
| Peripheral artery disease | 48 (6.0) | 9 (5.1) | 39 (6.3) | .580 |
| Previous stroke/transient ischemic attack | 100 (12.5) | 20 (11.4) | 80 (12.9) | .613 |
| Chronic obstructive pulmonary disease/asthma | 107 (13.4) | 17 (9.7) | 90 (14.5) | .103 |
| Sleep apnea/hypopnea | 65 (8.7) | 12 (8.1) | 53 (8.8) | .792 |
| Anemia | 200 (25.1) | 30 (17.1) | 170 (27.3) | .006 |
| Dementia | 31 (3.9) | 8 (4.6) | 23 (3.7) | .597 |
| Peptic ulcer disease | 38 (4.8) | 7 (4.0) | 31 (5.0) | .589 |
| Chronic liver disease | 27 (3.4) | 4 (2.3) | 23 (3.7) | .362 |
| Cancer history | 115 (14.4) | 21 (12.0) | 94 (15.1) | .301 |
| Risk scales | ||||
| CHA2DS2-VASc score | 3.4±1.8 | 3.3±1.7 | 3.4±1.9 | .632 |
| HAS-BLED score | 1.8±1.2 | 1.6±1.0 | 1.8±1.2 | .030 |
| Charlson score | 2.4±2.3 | 2.1±2.0 | 2.5±2.3 | .056 |
| Atrial fibrillation characteristics | ||||
| Type of atrial fibrillation | ||||
| First diagnosis | 68 (8.6) | 21 (12.1) | 47 (7.6) | .008 |
| Paroxysmal | 297 (37.7) | 47 (27.2) | 250 (40.7) | |
| Persistent | 113 (14.3) | 33 (19.1) | 80 (13.0) | |
| Long-standing persistent | 32 (4.1) | 9 (5.2) | 23 (3.7) | |
| Permanent | 278 (35.3) | 63 (36.4) | 215 (35.0) | |
| European Heart Rhythm Association functional class | ||||
| 1 No symptoms | 387 (49.1) | 97 (56.4) | 290 (47.1) | .073 |
| 2a Mild symptoms | 218 (27.7) | 48 (27.9) | 170 (27.6) | |
| 2b Moderate symptoms | 99 (12.6) | 14 (8.1) | 85 (13.8) | |
| 3 Severe symptoms | 77 (9.8) | 11 (6.4) | 66 (10.7) | |
| 4 Disabling symptoms | 7 (0.9) | 2 (1.2) | 5 (0.8) | |
| Previous procedures | ||||
| Electrical cardioversion | 163 (20.5) | 38 (21.7) | 125 (20.1) | .639 |
| Pulmonary vein ablation | 61 (7.7) | 3 (1.7) | 58 (9.3) | .001 |
| Arrhythmia surgery | 10 (1.3) | 1 (0.6) | 9 (1.4) | .358 |
| Atrioventricular node ablation | 13 (1.6) | 2 (1.1) | 11 (1.8) | .564 |
| Left atrial appendage percutaneous closure | 5 (0.6) | 0 (0.0) | 5 (0.8) | .234 |
| Pacemaker/resynchronization therapy/Implantabledefibrillator | 105 (13.2) | 21 (12.0) | 84 (13.5) | .603 |
| Physical exam | ||||
| Body mass index, kg/m2 | 29±5 | 28±5 | 29±5 | .347 |
| Systolic pressure, mmHg | 128±21 | 130±22 | 128±20 | .386 |
| Diastolic artery pressure, mmHg | 75±13 | 78±13 | 74±13 | .022 |
| Baseline heart rate, bpm | 75±18 | 74±19 | 75±17 | .577 |
| Complementary tests | ||||
| Sinus rhythm at inclusion visit | 322 (42.9) | 76 (46.9) | 246 (41.8) | .248 |
| Bundle brunch block | ||||
| No | 588 (78.5) | 129 (81.6) | 459 (78.5) | .461 |
| Right bundle branch block | 69 (9.2) | 14 (8.9) | 55 (9.3) | |
| Left bundle branch block | 77 (13.0) | 92 (12.3) | 15 (9.5) | |
| Left atrial enlargement | 463 (68.2) | 117 (78.0) | 346 (65.4) | .003 |
| Left ventricle ejection fraction | 57±13 | 59±9 | 56±13 | .02 |
| Anticoagulation prescribed in first visit | ||||
| No | 98 (12.3) | 22 (12.6) | 76 (12.2) | .174 |
| Vitamin K oral anticoagulants | 239 (30.0) | 41 (23.4) | 198 (31.8) | |
| Direct oral anticoagulants | 454 (57.0) | 111 (63.4) | 343 (55.1) | |
| Low molecular weight heparin | 6 (0.8) | 1 (0.6) | 5 (0.8) | |
| Concomitant treatment | ||||
| Antiarrhythmic drugs | 218 (27.4) | 30 (17.1) | 188 (30.2) | .001 |
| Platelet aggregation inhibitors | 100 (12.5) | 18 (10.3) | 82 (13.2) | .307 |
| Digoxin | 87 (10.9) | 18 (10.3) | 69 (11.1) | .762 |
| Beta-blockers | 544 (68.3) | 119 (68.0) | 425 (68.3) | .934 |
| Verapamil/diltiazem | 47 (5.9) | 12 (6.9) | 35 (5.6) | .542 |
| ACEI/angiotensin receptor blockers/sacubitril-valsartan | 442 (55.5) | 111 (63.4) | 331 (53.2) | .016 |
| Statins | 410 (51.4) | 87 (49.7) | 323 (51.9) | .605 |
| Proton pump inhibitors | 358 (44.9) | 79 (45.1) | 279 (44.9) | .946 |
| Nonsteroidal antiinflammatory drugs | 34 (4.3) | 4 (2.3) | 30 (4.8) | .142 |
ACEI, angiotensin conversing enzyme inhibitors.
Quantitative data are shown as mean±standard deviation, and qualitative data as No. (%).
A total of 797 patients were included in the study (age 72±11 years, 60% men figure 1), representing 100% of all patients with AF attended during the inclusion week. Most participants were outpatients at the time of inclusion. The most common risk factors were a sedentary lifestyle and hypertension. Over half of the patients had heart disease, with the most frequent being valvular heart disease, followed by ischemic heart disease. Nearly 25% had a history of heart failure, and 6.4% had at least moderate mitral stenosis or a mechanical prosthesis. Common comorbidities included renal failure and anemia. The average CHA2DS2-VASc, HAS-BLED, and Charlson scores were 3.4±1.8, 1.8±1.2, and 2.4±2.3, respectively. Most patients had paroxysmal or permanent AF and were either asymptomatic or mildly symptomatic. The mean ejection fraction was within the normal range, and most patients had left atrial dilation. Anticoagulation therapy was prescribed in 87.7% of patients, with 57% receiving direct oral anticoagulants. The most common concomitant treatment was beta-blockers, and 27.4% of patients were prescribed antiarrhythmic drugs (table 2).
Central illustration. ESC quality indicators in AF management in 9 Spanish centers with SEC-EXCELENTE in AF accreditation. AAD, antiarrhythmic drugs; AF, atrial fibrillation; ESC, European Society of Cardiology; ESRD, end-stage renal disease; OAC, oral anticoagulation; SEC, Spanish Society of Cardiology (Sociedad Española de Cardiología); TIA, transient ischemic attack.
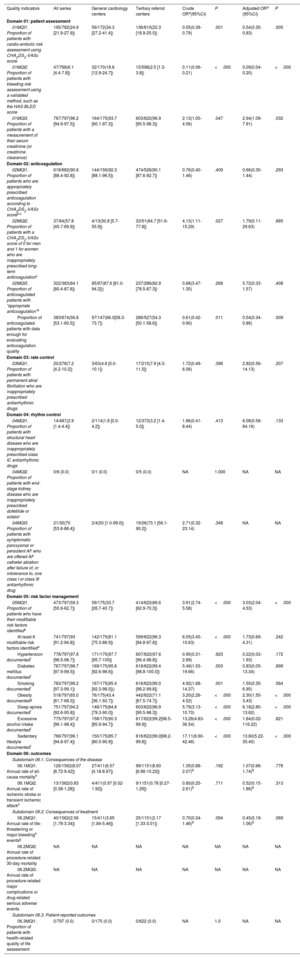
The ESC Qis are detailed in table 3 and figure 1. In domain 1 (patient assessment), the highest indicator was the documentation of renal function (96.2%), while documentation of CHA2DS2-VASc and HAS-BLED scores was low (24.9%) and very low (6.1%), respectively. Nevertheless, the variables needed to calculate these scores were available in all patients. In domain 2 (anticoagulation), a high rate of appropriate anticoagulation in high-risk patients and a high quality of anticoagulation were observed. Conversely, the high rate of anticoagulation in low-risk patients was notable, and anticoagulation quality was assessed in just over half of the patients.
Quality indicators of the European Society of Cardiology in centers with SEC-EXCELENTE in atrial fibrillation accreditation in Spain
| Quality indicators | All series | General cardiology centers | Tertiary referral centers | Crude ORa(95%CI) | P | Adjusted ORa (95%CI) | P |
|---|---|---|---|---|---|---|---|
| Domain 01: patient assessment | |||||||
| 01MQI1. Proportion of patients with cardio-embolic risk assessment using CHA2DS2-VASc score | 195/782(24.9 [21.9-27.9]) | 59/172(34.3 [27.2-41.4]) | 136/610(22.3 [18.9-25.5]) | 0.55(0.39-0.79) | .001 | 0.54(0.35-0.83) | .005 |
| 01MQI2. Proportion of patients with bleeding risk assessment using a validated method, such as the HAS-BLED score | 47/768(6.1 [4.4-7.8]) | 32/170(18.8 [12.9-24.7]) | 15/598(2.5 [1.3-3.8]) | 0.11(0.06-0.21) | <.000 | 0.09(0.04-0.20) | <.000 |
| 01MQI3. Proportion of patients with a measurement of their serum creatinine (or creatinine clearance) | 767/797(96.2 [94.9-97.5]) | 164/175(93.7 [90.1-97.3]) | 603/622(96.9 [95.5-98.3]) | 2.13(1.00-4.56) | .047 | 2.94(1.09-7.91) | .032 |
| Domain 02: anticoagulation | |||||||
| 02MQI1. Proportion of patients who are appropriately prescribed anticoagulation according to CHA2DS2-VASc scoreb,c | 618/682(90.6 [88.4-92.8]) | 144/156(92.3 [88.1-96.5]) | 474/526(90.1 [87.6-92.7]) | 0.76(0.40-1.46) | .409 | 0.66(0.30-1.44) | .293 |
| 02MQI2. Proportion of patients with a CHA2DS2-VASc score of 0 for men and 1 for women who are inappropriately prescribed long-term anticoagulationc | 37/64(57.8 [45.7-69.9]) | 4/13(30.8 [5.7-55.9]) | 33/51(64.7 [51.6-77.8]) | 4.13(1.11-15.29) | .027 | 1.79(0.11-29.63) | .685 |
| 02MQI3. Proportion of anticoagulated patients with “appropriate anticoagulation”d | 322/383(84.1 [80.4-87.8]) | 85/97(87.6 [81.0-94.2])) | 237/286(82.9 [78.5-87.3]) | 0.68(3.47-1.35) | .268 | 0.72(0.33-1.57) | .408 |
| Proportion of anticoagulated patients with data enough for evaluating anticoagulation quality | 383/674(56.8 [53.1-60.5]) | 97/147(66.0[58.3-73.7]) | 286/527(54.3 [50.1-58.6]) | 0.61(0.42-0.90) | .011 | 0.54(0.34-0.86) | .009 |
| Domain 03: rate control | |||||||
| 03MQI1. Proportion of patients with permanent atrial fibrillation who are inappropriately prescribed antiarrhythmic drugs | 20/278(7.2 [4.2-10.2]) | 3/63(4.8 [0.0-10.1]) | 17/215(7.9 [4.3-11.5]) | 1.72(0.49-6.06) | .396 | 2.82(0.56-14.13) | .207 |
| Domain 04: rhythm control | |||||||
| 04MQI1. Proportion of patients with structural heart disease who are inappropriately prescribed class IC antiarrhythmic drugs | 14/487(2.9 [1.4-4.4]) | 2/114(1.8 [0.0-4.2]) | 12/373(3.2 [1.4-5.0]) | 1.86(0.41-8.44) | .413 | 6.08(0.58-64.16) | .133 |
| 04MQI2. Proportion of patients with end-stage kidney disease who are inappropriately prescribed dofetilide or sotalol | 0/6 (0.0) | 0/1 (0.0) | 0/5 (0.0) | NA | 1.000 | NA | NA |
| 04MQI3. Proportion of patients with symptomatic paroxysmal or persistent AF who are offered AF catheter ablation after failure of, or intolerance to, one class I or class III antiarrhythmic drug | 21/30(70 [53.6-86.4]) | 2/4(50 [1.0-99.0]) | 19/26(73.1 [56.1-90.2]) | 2.71(0.32-23.14) | .348 | NA | NA |
| Domain 05: risk factor management | |||||||
| 05MQI1. Proportion of patients who have their modifiable risk factors identifiede | 473/797(59.3 [55.9-62.7]) | 59/175(33.7 [26.7-40.7]) | 414/622(66.6 [62.9-70.3]) | 3.91(2.74-5.58) | <.000 | 3.03(2.04-4.53) | <.000 |
| At least 6 modifiable risk factors identifiede | 741/797(93 [91.2-94.8]) | 142/175(81.1 [75.3-86.9]) | 599/622(96.3 [94.8-97.8]) | 6.05(3.45-10.63) | <.000 | 1.73(0.69-4.31) | .242 |
| Hypertension documentedf | 778/797(97.6 [96.5-98.7]) | 171/175(97.7 [95.7-100]) | 607/622(97.6 [96.4-98.8]) | 0.95(0.31-2.89) | .923 | 0.22(0.03-1.93) | .172 |
| Diabetes mellitus documentedf | 787/797(98.7 [97.9-99.5]) | 169/175(95.6 [92.6-98.6]) | 618/622(99.4 [98.8-100.0]) | 5.49(1.53-19.66) | .003 | 0.83(0.05-13.34) | .899 |
| Smoking documentedf | 783/797(98.2 [97.3-99.1]) | 167/175(95.4 [92.3-98.5])) | 616/622(99.0 [98.2-99.8]) | 4.92(1.68-14.37) | .001 | 1.55(0.35-6.95) | .564 |
| Obesity documentedg | 518/797(65.0 [61.7-68.3]) | 76/175(43.4 [36.1-50.7]) | 442/622(71.1 [67.5-74.7]) | 3.20(2.26-4.52) | <.000 | 2.30(1.55-3.43) | <.000 |
| Sleep apnea documentedf | 751/797(94.2 [92.6-95.8]) | 148/175(84.6 [79.3-90.0]) | 603/622(96.9 [95.5-98.3]) | 5.79(3.13-10.70) | <.000 | 6.18(2.80-13.62) | <.000 |
| Excessive alcohol intake documentedf | 775/797(97.2 [96.1-98.4]) | 158/175(90.3 [85.9-94.7]) | 617/622(99.2[98.5-99.9]) | 13.28(4.83-36.54) | <.000 | 1.64(0.02-116.22) | .821 |
| Sedentary lifestyle documentedf | 766/797(96.1 [94.8-97.4]) | 150/175(85.7 [80.5-90.9]) | 616/622(99.0[98.2-99.8]) | 17.11(6.90-42.46) | <.000 | 13.60(5.22-35.40) | <.000 |
| Domain 06: outcomes | |||||||
| Subdomain 06.1. Consequences of the disease | |||||||
| 06.1MQI1. Annual rate of all-cause mortalityh | 126/1562(8.07 [6.72-9.42]) | 27/411(6.57 [4.18-8.97]) | 99/1151(8.60 [6.98-10.22]) | 1.35(0.88-2.07)9 | .192 | 1.07(0.66-1.74)8 | .776 |
| 06.1MQI2. Annual rate of ischemic stroke or transient ischemic attackh | 13/1562(0.83 [0.38-1.28]) | 4/411(0.97 [0.02-1.92]) | 9/1151(0.78 [0.27-1.29]) | 0.80(0.25-2.61)9 | .711 | 0.52(0.15-1.86)8 | .313 |
| Subdomain 06.2. Consequences of treatment | |||||||
| 06.2MQI1. Annual rate of life-threatening or major bleedingh eventsg | 40/1562(2.56 [1.78-3.34]) | 15/411(3.65 [1.84-5.46]) | 25/1151(2.17 [1.33-3.01]) | 0.70(0.34-1.46)9 | .094 | 0.45(0.19-1.06)8 | .069 |
| 06.2MQI2. Annual rate of procedure-related 30-day mortality | NA | NA | NA | NA | NA | NA | NA |
| 06.2MQI3. Annual rate of procedure-related major complications or drug-related serious adverse events | NA | NA | NA | NA | NA | NA | NA |
| Subdomain 06.3. Patient-reported outcomes | |||||||
| 06.3MQI1. Proportion of patients with health-related quality of life assessment | 0/797 (0.0) | 0/175 (0.0) | 0/622 (0.0) | NA | 1.0 | NA | NA |
95%CI, 95% confidence interval; NA, not available; OR: odds ratio.
All values are expressed as numerator/valid denominator (percentage [95% confidence interval]).
Appropriateness of anticoagulation prescription was defined as CHA2DS2-VASc score of ≥ 1 for men and ≥ 2 for women as in the 2020 ESC Guidelines.
Anticoagulation was considered “appropriate” if time in therapeutic range was at least 70% for vitamin K antagonists, or the dose was correct according to the manufacturer's recommendations for direct anticoagulants.
Modifiable risk factors: hypertension, diabetes mellitus, smoking, obesity, sleep apnea, excessive alcohol intake, and sedentary lifestyle.
In domains 3 and 4 (rate and rhythm control), inappropriate use of antiarrhythmics was low, and more than 70% of symptomatic patients with paroxysmal or persistent AF were offered ablation after failure or intolerance of antiarrhythmics, although this QI could only be assessed in 30 patients (). In domain 5, all 7 modifiable risk factors were identified, as proposed by the ESC, in only 59.3% of the patients. When analyzed individually, all factors were included in >90% of reports, except for obesity: weight and height were recorded in the clinical history in only 65% of patients.
Finally, in domain 6 (outcomes), the annual rates of total mortality, ischemic stroke/transient ischemic attack, and major bleeding were 8.1 (n=126 patients), 0.8 (n=13 patients), and 2.56 (n=33 patients) per 100-patients/y, respectively. Vital status was known in all patients at the end of follow-up; 33% (42/126) of deaths were due to cardiovascular causes, and 12 patients died from unknown causes. The registry did not include data on mortality or major complications related to procedures or severe adverse events related to medications. Additionally, none of the centers used validated health-related quality of life scales in routine clinical practice.
Baseline features of patients and quality indicators in AF of the ESC according to the center complexityA total of 3 hospitals were classified as general cardiology centers, and 6 were tertiary referral centers. Tertiary referral centers had significantly more cardiologists, a greater number of total hospitalization beds, more cardiology hospital discharges, and more outpatient clinic visits. These centers also performed a significantly higher number of percutaneous cardiac structural procedures, cardiac electronic device implantations, and AF ablation procedures ().
Patients in tertiary referral centers were more frequently hospitalized at the time of inclusion and exhibited a higher prevalence of sedentary lifestyle, anemia, paroxysmal AF, previous pulmonary vein ablation, and antiarrhythmic drug prescriptions. They also had higher HAS-BLED scores and showed a trend toward higher Charlson indices, more severe symptoms, and a higher frequency of coronary surgery and mitral stenosis or mechanical valve prosthesis. Conversely, the frequency of atrial enlargement was lower in this group; these patients had a lower left ventricular ejection fraction, and renin-angiotensin system blockers were less frequently prescribed (table 2).
In general cardiology centers, CHA2DS2-VASc and HAS-BLED scores were more frequently documented, and inappropriate anticoagulation in low-risk patients was less common. Conversely, renal function and all modifiable risk factors were more frequently documented in tertiary referral centers. Despite these differences, both groups had a similar and high proportion of high-risk patients prescribed anticoagulation and those with appropriate anticoagulation. However, data availability for evaluating the quality of anticoagulation was higher in general cardiology centers. No significant differences were observed in QI in other domains.
After multivariate adjustment for unbalanced baseline features, the differences in inappropriate anticoagulation for low-risk patients disappeared. Discrepancies in the assessment of modifiable risk factors were primarily related to obesity, sedentary lifestyle, and sleep apnea, which were more frequently documented in tertiary referral centers. No significant differences were found in the outcomes domain QIs between the groups after adjustment (table 3).
DISCUSSIONThe main findings of this study are that, in centers with SEC-EXCELENCE in AF accreditation in Spain, the results for several key QI were acceptable, particularly in the domains of outcomes, rate control, rhythm control, and select aspects of patient assessment and anticoagulation. However, worse values were found in areas such as documentation of cardioembolic and bleeding risk, anticoagulation in low-risk patients, risk factor management, and patient-reported outcomes, suggesting a need for significant improvement in these areas. Notably, most QI values were similar, independently of the complexity of the center, with few exceptions, mainly in patient assessment and risk factor management domains, but not in the outcomes domain.
Previous studies assessing quality indicatorsThree previous studies have examined the QI in AF proposed by the ESC.4–6 The BALKAN-AF survey included 2712 patients from 49 centers in 7 Balkan countries from December 2014 to February 2015, but without follow-up.4 The ChiOTEAF registry included 6420 patients aged ≥65 years, with 1-year follow-up, in a prospective registry conducted between October 2014 and December 2018 in 44 centers in China.5 The Danish AF registry evaluated several ESC QI in >100 000 patients from 2017 to 2021.6 This national-scale registry based on administrative databases includes nearly all Danish AF patients, except those exclusively attended in private cardiology or primary care facilities.
While these registries provide a strong foundation for understanding QI assessment in AF, they have limitations. The BALKAN-AF study could not assess outcome domain QIs, and results from the ChiOTEAF and Danish AF registries may not be directly applicable to Spain due to differences in racial characteristics, health care systems, and access to anticoagulants. Previous studies in Spain, published in 2012 and 2016, reported care indicators in 160 and 533 patients from 1 and 2 tertiary Spanish hospitals, respectively, but were conducted before the ESC QIs were available21,22 and lacked follow-up data. Moreover, these studies did not explore the possible association of the type of center with the QI in patients with AF. Therefore, our study provides a contemporary evaluation of the QI proposed by the ESC, including the outcome domain, in a Spanish sample including centers of varying complexity.
Quality indicators: patient assessment and anticoagulationRenal function was documented in 96.2% of patients, which is higher than the BALKAN-AF4 and ChiOTEAF5 registries (76.1% and 81.5%, respectively) and similar to the Danish AF registry6 (93%). A possible reason for these discrepancies is the different timing of recruitment, as the implementation of recommendations is expected to improve over time. Our low rates of recording cardioembolic and bleeding risk scores compare unfavorably with other registries, which report figures of more than 90%.4,5 However, the proportion of patients receiving anticoagulation among those with high cardioembolic risk was 90.6%, similar to the 90.4% reported by the Danish registry in 2021, and higher than the 74.4% and 44.7% reported by other registries.4,5 Although the data from the BALKAN AF4 registry could be explained by the timing of inclusion (the Danish AF registry observed an increase in this proportion from 85.3% in 2017 to 90.4% in 2021), the 44.7% reported by the ChiOTEAF registry suggests that other factors may affect the underuse of anticoagulants in this population. The high proportion of anticoagulation in low-risk patients in our series (57.8%) has also been reported in other studies (39%-60%).4,5,21 Some of these patients could be in the pre- or postcardioversion or postablation period, but therapeutic inertia or the low rate of documentation of the CHA2DS2-VASc score, combined with the mistaken perception of higher embolic than bleeding risk, could contribute to this issue. Although all efforts were made by investigators to avoid missing any embolic risk factors during data collection, it is impossible to ensure that this was not the case in some instances.
Quality indicators: rate and rhythm controlAntiarrhythmic drugs were inappropriately prescribed in 7.2% of patients with permanent AF, which is similar to the percentages reported in other studies (3.6% and 10%).4,5 No patients with end-stage kidney disease received dofetilide or sotalol, and 2.9% of those with structural heart disease were prescribed Class IC antiarrhythmic drugs, which is consistent with previous reports (0.7% and 2.2%).4,5 Although these QI values are low, they represent a clear opportunity for improvement. None of the previous registries assessed the proportion of patients with symptomatic paroxysmal or persistent AF who were resistant to or intolerant ≥1 class I or III antiarrhythmic drug and were offered catheter ablation. While the 70% observed in our study is promising, it should be interpreted with caution due to the clearly insufficient sample size to draw solid conclusions
Quality indicators: risk factor managementMost risk factors were correctly documented, except for obesity: weight and height were recorded in in the electronic medical record in only 65% of patients. A reporting bias for obesity (ie, weight and height being more frequently registered in obese patients) could partly explain the high obesity rate in our sample (37.3%), which is higher than that reported in other studies (5.4% and 24.7%).4,5 Other series including consecutive AF patients attended in cardiology outpatient clinics in Spain have reported a prevalence of obesity ranging from 10% to 26.2%.22,23 However, another Spanish study described a body mass index of 29.2±3.9kg/m2, which is nearly identical to the 29±5kg/m2 found in our series. Notably, the prevalence of obesity in the general population in Spain was 15.7% in 2020,24 suggesting that it is unlikely to be lower in AF patients. For the other risk factors, the prevalence in our study was similar to that in previous studies.4,5,21–23,25
Quality indicators: outcomesThe rate of stroke/transient ischemic attack in our study (0.83/100-patients/y) was identical to that reported by the Danish AF registry for 2019 (0.83/100-patients/y),6 and similar to another contemporary Spanish study (1.07/100-patients/y)23 and the ChiOTEAF registry (1.1/100-patients/y).5 The rate of major bleeding was 2.23/100-patients/y for 2019 in the Danish registry,6 which is very similar to the rate in our study (2.56/100-patients/y). However, this rate was 1.6/100-patients/y in the ChiOTEAF registry,5 possibly related to the lower anticoagulation rate in that work. For all-cause mortality, our rate (8.07/100-patients/y) was similar to those of other Spanish series (8.24/100-patients/y),23 but slightly higher than that reported by the ChiOTEAF registry (6.8/100-patients/y).5 This last result is somewhat surprising, given that the median age in our study was 76 years, compared with a mean age of 72 years in our work and 73.8 years in the other Spanish registry.23 However, the 95% confidence interval for this QI in our series [6.72-9.42/100-patients/y] included the rate reported by the ChiOTEAF registry.5 In all 3 studies, most deaths were noncardiovascular.
Validated health-related quality of life scales were not used in routine clinical practice in our study. The BALKAN-AF study4 reported the frequency of “patient-reported outcomes”, but the description of the data suggested that these outcomes were mainly “physician perceived symptoms”. In contrast, the ChiOTEAF registry5 measured quality of life outcomes using visual analog scales. The 2020 ESC AF guidelines recommend routine collection of patient-reported outcomes,2 but this objective should be achieved using validated tools.3 Implementing these measurements in daily clinical practice will clearly be a challenge for health care centers in the coming years.26
Assessment of quality indicators by center complexityTo the best of our knowledge, the present study is the first to describe results by center complexity in the AF process. Although some differences were observed in the domains of patient evaluation and risk factor management, the QIs for anticoagulation and outcome domains were not significantly different, suggesting that general cardiology centers can achieve the same quality of care in the AF process as tertiary referral centers. Notably, the inclusion period preceded the publication of the QIs. However, although physicians were not aware of them at the time, many of the QIs of the SEC-EXCELENTE in AF7 overlap with those of the ESC, which could partly explain the observed results.
Clinical implicationsThe main clinical implication of this work is that it highlights the importance of evaluating the quality of care using robust indicators, such as those proposed by the ESC. Identifying opportunities to improve the quality of care is the only way to address gaps in patient management. We believe that systematically monitoring QIs, as proposed by the ESC guidelines, could be an effective way to achieve this objective, both at a national and European level.
LimitationsThe main limitation of this study is that the sample size was insufficient to precisely estimate some of the QIs (eg, ablation offered to symptomatic patients with AF), and therefore these results must be interpreted with caution. In addition, details of clinical events were not revised by an independent committee, nor was an external audit performed. Furthermore, the inclusion of patients treated by cardiologists exclusively limits our conclusions to this specialty. In addition, these data represent the performance of 9 centers with an active interest in improving AF management, which may not be representative of national health care in Spain. Therefore, comparisons with other registries that are nearly all-inclusive, such as the Danish registry,6 are applicable only to this set of cardiology units. The causes of 12 deaths were unknown, and consequently the true number of events may be underestimated. Moreover, hospital admissions in private centers could have been missed as events. Equally, we were unable to evaluate the secondary QIs proposed by the ESC. Finally, we were unable to assess the true adherence of patients with prescribed medications or follow-up, as we only had access to physicians’ prescription at the first visit.
CONCLUSIONSAlthough accredited centers in Spain demonstrated good performance in many of the ESC QIs for AF, and general cardiology and tertiary referral hospitals showed similar results in the anticoagulation and outcomes domains, there is still room for improvement. This information could serve as a starting point for the participating centers to identify areas for improvement and work toward achieving excellence in the care of patients with AF.
- -
AF is the most common cardiac arrhythmia and is associated with substantial morbidity and mortality.
- -
The ESC proposed a set of QI for AF in its 2020 guidelines.
- -
These QI have been assessed in several populations outside Spain.
- -
This is the first study to assess the ESC QIs for AF in Spain.
- -
The anticoagulation and outcome QIs were similar to or better than those reported in other European countries.
- -
Similar results were found among general cardiology and tertiary referral centers for anticoagulation and outcome QIs.
The Spanish Society of Cardiology provided the online database for collecting data and financed the contract research organization for management of administrative authorizations and support of the study process.
ETHICAL CONSIDERATIONSThe study protocol was approved by the Research Ethics Committee of each center and complied with the recommendations for medical studies outlined in the Declaration of Helsinki. Since the data used in the study were purely clinical-care related, anonymous, and dissociated from personal information, the Research Committees approved a waiver of informed consent. The SAGER guidelines on sex/gender reporting were followed.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used to write any section of this manuscript.
AUTHORS’ CONTRIBUTIONSConceptualization, formal analysis, writing—original draft preparation, supervision, project administration, and funding acquisition were carried out by M. Ruiz Ortiz. Investigation was conducted by all authors. Writing—review and editing were performed by all other authors. All authors have approved the final version of the manuscript for publication and are accountable for all aspects of the work.
CONFLICTS OF INTERESTNone.
The authors warrant that the following investigators are responsible for the data contained in this manuscript:
Hospital Universitario Reina Sofía, Córdoba, Spain: Martín Ruiz Ortiz.
Hospital Universitario La Paz, Madrid, Spain: Rafael Peinado Peinado.
Hospital Clinic, Barcelona, Spain: Elena Arbelo Laínez.
Hospital Costa Del Sol, Marbella (Málaga), Spain: Almudena Valle Alberca.
Hospital General Universitario de Alicante, Alicante, Spain: Alicia Ibáñez Criado.
Hospital Marina Salud, Denia, Alicante, Spain: Alfonso Valle Muñoz.
Hospital Universitario y Politécnico La Fe, Valencia, Spain: Joaquín Osca Asensi.
Hospital Universitario de Jerez de La Frontera, Cádiz, Spain: Ana del Río Lechuga.
Hospital Universitario Virgen de la Victoria, Málaga, Spain: Alejandro I. Pérez Cabeza.