Currently, studies on the leadless pacemaker (Micra) have mostly been limited to clinical trials with less than 6 months’ follow-up and they often fail to reflect real population outcomes. We sought to evaluate electrical parameters at implantation and chronologically during follow-up, as well as the safety of this new technique.
MethodsThis prospective, observational study included 30 consecutive patients, all ≥ 65 years, with an indication for single-chamber pacemaker implantation.
ResultsSuccessful implantation was accomplished in all patients referred for leadless implantation. The mean age was 79.4±6.4 years (range, 66-89 years); 20 (66.6%) were men and 28 had permanent atrial fibrillation (93.3%); 1 had atrial tachycardia and 1 had sinus rhythm. Concomitant atrioventricular node ablation was performed immediately after implantation in 5 patients (16.6%), and implantation was performed after transcatheter aortic valve implantation in 2. The procedure was performed under an uninterrupted anticoagulation regimen (maximum INR 2.4) in 23 patients (76.6%). With the exception of 1 moderate pericardial effusion without tamponade, there were no severe complications. The mean follow-up was 5.3±3.3 months and 4 patients had more than 1 year of follow-up. Sensing and pacing parameters were stable both at implantation and during the short- to mid-term follow-up.
ConclusionsImplantation of leadless pacemakers is feasible, safe and provides advantages over the conventional system. Further studies with longer follow-up periods will be needed before these devices become widely used in routine clinical practice.
Keywords
Due in part to population aging, current medical practice is witnessing a considerable increase in the number of pacemakers implanted, which has led to a rise in the complications associated with these devices.1 The overall complication rate is estimated at 6% to 10% and includes those related to the implantation procedure (pneumothorax), pericardial effusion, cardiac perforation, problems with the generator pocket (hematoma, infection, skin erosion), lead complications (fracture, displacement, insulation breaches), and sometimes, venous occlusion.2–6
Within this scenario, leadless pacemaker systems have emerged, which in theory could overcome some of the complications associated with conventional systems. Nonetheless, the available data on the safety and efficacy of leadless pacemakers has mainly come from clinical trials.7–9 There is much less evidence from patients in real-life practice.
The aim of this study was to evaluate the electric parameters at implantation and over follow-up of the Micra leadless transcatheter pacemaker (Medtronic Ibérica, SA), and to report on the potential indications of the system and possible complications related to the implantation procedure.
METHODSThis prospective, observational study included 30 consecutive patients (June 17, 2015 to May 10, 2016) with an indication for single-chamber pacemaker placement and age ≥ 65 years. The implantation procedure was carried out using a femoral approach and conventional technique.7 Target parameters at implantation were as follows: pacing threshold ≤ 1.0V to 0.24ms, pacing impedance 400 to 1500Ω, and R wave amplitude ≥ 5mV. All patients underwent transthoracic echocardiography, chest radiography, and electrocardiography following the procedure. Informed consent was obtained from all patients.
In accordance with Reynolds et al.,9 a major adverse event was defined as one leading to death or serious deterioration of the patient's clinical condition, an event producing a vital risk and requiring some type of intervention for resolution, and any complication that prolonged hospital admission more than 48hours.
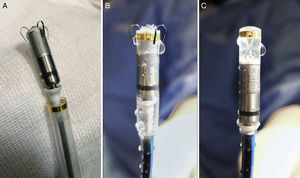
Micra Pacemaker Implantation MethodThe Implantable Micra DeviceThe percutaneous Micra pacemaker (MC1VR01 Medtronic) is a miniaturized, single-chamber transcatheter pacemaker system that provides bipolar sensing and pacing to the right ventricle. Micra is contained in a hermetically sealed capsule whose volume is 0.8 cm,3 length 25.9 mm, external diameter 6.7 mm, and weight 2.0 g. The device has an active fixation mechanism composed of 4 electrically inactive nitinol tines, designed to attach to the cardiac tissue at the right ventricular site selected for the implant (Figure 1). As to pacing, the cathode is a titanium-coated, sintered platinum, steroid-eluting electrode. At the opposite end, the device has a recessed retrieval feature to enable acute extraction with a lasso catheter.
Micra pacemaker and deployment system. A: view of the device after it is removed from the sterile package. B: view of the device as it is flushed with saline solution before complete retraction into the device cup (also how it will look at initiation of deployment). C: the device is completely retracted into the device cup, and is adequately perfused with heparinized saline solution before the delivery system is inserted in the introducer sheath.
Communication with the device is carried out by conventional radiofrequency telemetry using the Medtronic programmer. Micra is compatible with 3T magnetic resonance imaging and has an estimated service life of 10 years with 100% pacing at 60 bpm, 1.5 V output with pulse duration of 0.24 ms, and 600Ω impedance.
Introducer and Delivery CatheterA specific introducer sheath for femoral access, the Medtronic MI2355A, is used for implantation. The introducer is 55.7cm long, has an internal diameter of 23 Fr (external diameter, 27 Fr), and possesses a hydrophilic coating to facilitate insertion. Once the introducer is inserted, the patient receives a bolus dose of 3000 IU of heparin, and thereafter, continuous perfusion of heparinized saline solution through the introducer side-port. The delivery catheter is designed to position the device in the right ventricle, with access through the femoral vein. The catheter is 105cm long and has a deflectable, flexible 23-Fr body with a compartment at the distal end to contain the device (Figure 1). The Micra remains joined to the catheter during implantation by a tether that runs along the length of the catheter forming a loop from the delivery system handle to the distal device recapture cone, which enables recovery and repositioning of the device, if needed. The handle also allows adjustment of the tether to advance or retract the distal compartment for engaging and repositioning the device.
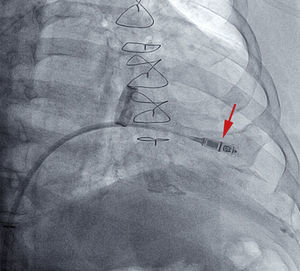
Once the steerable catheter is situated in the right ventricle, a site in the septal apical or medial septal region is sought and evaluated to determine its suitability for releasing and attaching the device. This is achieved by injecting contrast medium and filming in 2 complementary radiologic projections for further analysis ().
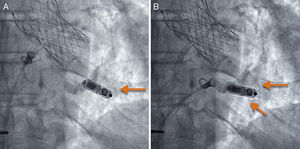
If the position is deemed adequate, the device is deployed, usually in a right oblique projection (Figure 2 and ). The electrical parameters of the device are then determined, and if they are appropriate, the degree of fixation is evaluated. The maneuver of pulling and holding for a few seconds is performed, and the movement of the device tines is filmed and then analyzed frame by frame. Fixation is considered adequate when movement of at least 2 of the 4 tines is confirmed (Figure 3 and ). Following the pull-hold maneuver, the electrical parameters are again determined and if they show no changes, implantation is considered complete. The tether threads are cut and withdrawn, and finally, the introducer is withdrawn and percutaneous closure is carried out using figure-8 sutures with No. 1 silk thread.
Pull and hold maneuver to evaluate the degree of fixation by putting tension on the system's tether. (A) Baseline image and (B) after gentle pulling and holding, which shows that 2 of the tines are curved outward from the device, the upper one being more markedly engaged (arrows).
All patients underwent electrocardiography and posterior-anterior and lateral chest radiography before hospital discharge (24 h following the procedure). At 1, 3, 6, and 12 months later, the automated measurements of the device were reviewed, and the adequacy of the programming and pacing parameters was confirmed manually.
Statistical AnalysisAn initial descriptive analysis was performed, in which the continuous Gaussian variables are expressed as the mean±standard deviation, and non-Gaussian variables as the median (range). Statistics were carried out with SPSS 19.
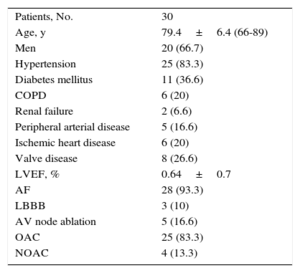
RESULTSBaseline CharacteristicsThe baseline characteristics of the patients are shown in Table.
Baseline Characteristics of the Patients
| Patients, No. | 30 |
| Age, y | 79.4±6.4 (66-89) |
| Men | 20 (66.7) |
| Hypertension | 25 (83.3) |
| Diabetes mellitus | 11 (36.6) |
| COPD | 6 (20) |
| Renal failure | 2 (6.6) |
| Peripheral arterial disease | 5 (16.6) |
| Ischemic heart disease | 6 (20) |
| Valve disease | 8 (26.6) |
| LVEF, % | 0.64±0.7 |
| AF | 28 (93.3) |
| LBBB | 3 (10) |
| AV node ablation | 5 (16.6) |
| OAC | 25 (83.3) |
| NOAC | 4 (13.3) |
AF, atrial fibrillation; AV, atrioventricular; COPD, chronic obstructive pulmonary disease; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NOAC, new oral anticoagulant; OAC, oral anticoagulant
Values are expressed as No. (%) or mean±standard deviation (range).
The outcome of the implantation procedure was satisfactory in all 30 patients selected. Mean age was 79.4±6.4 (66-89) years and 20 patients (66.7%) were men. Mean follow-up was 5.3±3.3 months. At the time of the procedure 28 of 30 patients had permanent atrial fibrillation (AF). The indications for pacemaker implantation were slow AF in 28 patients (93.3%), trifascicular block and syncope in 1 patient in sinus rhythm, who was expected to require a low percentage of pacing, and recurrent episodes of rapid atrial tachycardia in another patient. Of note, 2 patients underwent pacemaker implantation following percutaneous aortic valve implantation. One patient had a mechanical mitral valve and a De Vega tricuspid annuloplasty, and another had severe mitral regurgitation.
In 5 patients with inadequate control of the ventricular response (16.6%), radiofrequency ablation of the atrioventricular (AV) node to improve valve competence was performed in the same procedure, immediately after percutaneous implantation of the Micra pacemaker. Ablation used the same sheath, advancing with 16-Fr and 8-Fr introducers, and the additional time needed (mean, 5±2 minutes) did not significantly prolong the procedure.
Implantation was performed under uninterrupted oral anticoagulant (OAC) therapy with coumerins in 23 patients (76.6%); the maximum international normalized ratio (INR) was 2.4. In 4 other patients who were receiving new oral anticoagulant agents, the dose prior to pacemaker implantation was omitted and therapy was reinitiated on the same day as the procedure. Hence, heparin bridge therapy was not used in any patient.
The device was positioned in the apical region in 20 patients (66.6%), in the medial septal region in 9 (30%), and in the right ventricular outflow tract in 1 (3.3%).
ComplicationsPacemaker implantation was achieved in all patients, with a low rate of major complications, no displacements, and no systemic infections. Of note, 1 patient experienced a moderate pericardial effusion with no hemodynamic repercussions that was resolved conservatively and did not prolong the hospital stay. The patient was 83 years of age, was not receiving OACs, and the device was placed in the medial septal region. In another patient, the left femoral vein was used to implant the device because the right femoral vein showed excessive tortuosity and a local dissection. The patient was discharged 24hours after the procedure, with no local complications immediately after or during follow-up. No local venous access-related complications occurred in any of the patients.
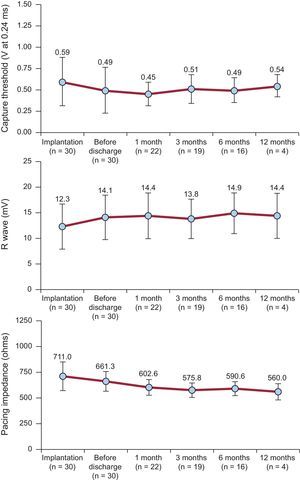
Follow-upThe pacing variables were excellent at implantation and at short- and mid-term follow-up (Figure 4). Four patients completed 1 year of follow-up with no changes in the sensing or pacing parameters, or in the pacing impedances.
DISCUSSIONIn this article, we describe our experience with transcatheter implantation of the Micra pacemaker. These are the first available data on the outcomes of patients receiving these devices in a large Spanish series, and the results support the findings published by other groups. The technique was safe and the system showed excellent immediate and short-term parameters, thus confirming in real-life practice the data obtained in clinical trials. Furthermore, the system provides several potential advantages over conventional pacemakers, such as the possibility of avoiding hematomas in patients receiving OAC treatment, elimination of generator pocket and lead infections, and the potential to carry out simultaneous AV node ablation.
Contextualizing the ProblemWith population aging, current medical practice is facing an increase in the number of pacemaker implantation procedures, which are associated with a far from negligible complication complications, such as infections and hematomas.2–6 These complications can also occur in device replacement procedures.5,10–13
The estimated complication rate in conventional pacemaker implantation ranges from 6% to 10%2,3 and increases to 14.2% when a new lead is implanted, the device must be checked for malfunctioning, or an upgrade of the pacing system is required.2
With regard to cardiac perforations, the rate associated with conventional pacemakers is 1%.2,14,15 The use of active fixation systems has been associated with a low perforation rate. In the study by Cano et al.,15 the rate of clinically relevant cardiac perforations was reported at only 0.8% and the predictors of this event were advanced age and female sex.
The use of the new Micra transcatheter pacemaker system could, at least theoretically, allow minimization of the complications associated with conventional systems, particularly those related to the 2 elements that are eliminated in the Micra: a) the pacing leads and connections, and b) the generator pocket implanted in the prepectoral region. Another factor to consider is the >10-year estimated service life of the device.16 This raises doubts about its use in patients with a long life expectancy, but experimental studies have determined that the right ventricle can accommodate up to 3 such devices.17–19 In addition, acute capture and extraction of the device at a few months of follow-up has been possible, but current knowledge on the amount of fibrosis associated with the implant is insufficient to predict the feasibility of later withdrawal.20–23
Analysis of the Series and Its ParticularitiesThe present series has several important particularities. In our opinion, the most important is that the procedure was carried out without interrupting OAC therapy, which did not imply a higher incidence of pericardial effusion or puncture site complications. This is an important, considering that in the pivotal trial8 anticoagulation was not used in 36% of patients vs 64% who received this therapy (heparin bridge therapy in 40% and OAC in 24%). In the study by Pachón et al.,24 7 of the 10 patients included were receiving OACs, but therapy was discontinued for the procedure in 5 of them.24 From our point of view, it seems safe to carry out the implantation procedure while continuing the anticoagulant regimen, a therapy known to be associated with hematomas when conventional pacemaker systems are used. Therefore, safe implantation could be performed in at-risk populations (eg, carriers of valvular prostheses) without the need for heparin bridge therapy.25–27 Among the patients in our series, 2 underwent Micra placement immediately after percutaneous valve implantation, 1 patient had a mitral valve prosthesis, and 2 others had AF and severe mitral regurgitation, all situations in which OAC interruption, even with heparin bridge therapy, would considerably increase the risk of complications.28
Second, for the first time, the versatility of the procedure was seen, as pacemaker implantation was combined with AV node ablation. In our experience (although limited to 5 patients), the combined objective was satisfactorily achieved by taking advantage of the introducer sheath of the Micra system. This is a clear advantage over conventional systems, since a second puncture and the additional cost of a second procedure are avoided. Nonetheless, it may be argued that this is a risky strategy. Based on our previous experience with implantation of this device, we believe that the combined technique could be a safe, convenient strategy when there is no device displacement or malfunctioning and patients are monitored by telemetry for a minimum of 24hours following the procedure. The combination strategy of AV node ablation and pacemaker implantation in the same procedure is now commonly performed in our center and there have been no complications related to dislodgement of the ventricular electrode. Nevertheless, the value of this approach should be confirmed in future studies with larger samples before it becomes widespread practice.
Third, we believe the present study provides clinically relevant information: Although the patients were drawn from clinical practice and we included the learning curve, there was a lower incidence of complications than that reported in the pivotal clinical trial.8 Specifically, that trial had 30 relevant complications (in 26 patients, 18.6%), including ventricular arrhythmia (n=9, 6.4%), femoral complications (n=9, 6.4%), and pericardial effusion without tamponade (n=1, 0.7%). In the study by Reynolds et al.,9 there were 28 major complications (in 26 patients, 3.2%), the most important being embolism/thrombosis (n=2, 0.3%), vascular complications (n=5, 0.7%), myocardial injury (n=11, 1.6%) and problems with the pacing system (n=2, 0.3%).
The total complication rate in the study by Reynolds et al.9 was only 4%, half the value reported in a historical series used for comparison (7.4%). Nonetheless, myocardial injury in patients receiving the Micra device was 1.6%, higher than the 1.1% in the comparison group. The predictors of this adverse event were advanced age, low weight, female sex, chronic obstructive pulmonary disease, steroid use, and previous percutaneous coronary artery revascularization. These are the factors classically associated with the risk of perforation related to the intravenous leads in conventional permanent pacemakers.9,14,15
There was 1 major complication in our series (pericardial effusion) and no major complications at the venous access site. Therefore, our data concur with those of Pachón et al.,24 who also reported an absence of notable complications following the procedure. These results may encourage more widespread implementation of this technique in other centers, providing that the patients selected are compatible with the characteristics of the device.
Follow-up of the Electrical ParametersAt the scheduled clinical follow-up visits (24 hours, and 1, 3, and 6 months after the procedure) there were no cases of device displacement or elevation of the pacing thresholds. Moreover, the 4 patients who completed 1 year of follow-up showed an identical trend. The trend in the electrical pacing parameters (Figure 4) was very good, supporting the data described in the study by Reynolds et al.9; that is, a decrease in the acute threshold in the first 24hours, with stable values during follow-up. The same pattern was observed in the impedance values. This information is of particular clinical importance because, together with the automatic capture control function, it enables the device to achieve an estimated service life of 8 to 9 years.
We found that the R wave detection values tended to increase slightly over follow-up. This is likely because this sequence has a relationship with the local microtrauma and injury associated with deployment of the device and the later beneficial effect of steroid elution from the cathode. This has sparked controversy in some forums,29,30 and it is certainly important to adhere to the recommendation to obtain values >5 mV. Nonetheless, we believe that borderline values may be acceptable considering the inherent risk involved in device repositioning. In the study reported by Pachón et al.,24 1 patient had an R wave value at implantation of 4.7 mV that increased to 5.7 mV during follow-up. In our series, 1 patient had excellent threshold and impedance values, and an R wave value of 4.4 mV at implantation, which increased to 5.5 mV in 24 hours. Thus, we believe that, in terms of pacing, it is much more important to have an optimal capture threshold (increased device duration) as well as good impedance of around 700-800Ω, which, in addition to favoring the longevity of the device, is an indirect marker of good fixation to the endocardium (and the safety of the implant).
LimitationsA limitation of this study is that it was a single-center, nonrandomized study. In addition, the number of patients included and the follow-up are relatively small. Although it provides 1-year follow-up data for the first time, only 4 patients completed this period; hence, there is a need for studies with longer follow-up and including larger numbers of patients. Last, only 2 professionals performed all the procedures; therefore, the results cannot be considered applicable to all centers.
CONCLUSIONSIn our experience, transcatheter implantation of the Micra pacemaker is a safe procedure associated with excellent immediate and short- to mid-term parameters that could avoid the complications associated with conventional devices. Furthermore, it can be safely performed in patients receiving anticoagulant therapy, and it allows AV node ablation in the same procedure through the device implantation system. Further studies are needed to confirm these findings and provide long-term data before the use of this device is generalized.
CONFLICTS OF INTERESTJ.L. Martínez-Sande is a proctor for the Micra pacemaker.
- –
Conventional pacemaker systems are associated with nonnegligible rates of acute and chronic complications which, theoretically, could be avoided with the new leadless intracardiac pacemaker devices.
- –
According to the data from clinical trials and small retrospective series, transcatheter implantation of the Micra pacemaker has an acceptable safety profile.
- –
This system could be an attractive alternative for specific patient subgroups with indications for intracardiac pacemaker implantation, such as those with venous access problems, a risk of hematoma or infection, or congenital heart disease.
- –
The experience of transcatheter implantation of the Micra pacemaker are reported in a real-life cohort of patients.
- –
Safety data are provided, not only related to the device, but also to the possibility of performing the procedure without interrupting OAC therapy in patients at high risk of thromboembolism.
- –
The feasibility of performing transcatheter pacemaker implantation and AV node ablation in the same procedure are reported for the first time.
- –
Follow-up results at 1-year postimplantation are reported, which show excellent pacing and sensing parameters during that time.