In patients with tricuspid regurgitation (TR), edge-to-edge transcatheter tricuspid valve repair (TTVR) is the strategy with the highest penetration worldwide. A dedicated edge-to-edge TTVR system has recently become available in Europe. The present study describes the initial experience with the system in Spain.
MethodsThis multicenter study collected individual data from the centers accepted for the use of the novel system within an initial limited release. Between June 2020 and March 2021, all patients undergoing an edge-to-edge TTVR using the TriClip system in Spain were included in the study. The primary endpoint was the achievement of a TR reduction of at least 1 grade at discharge.
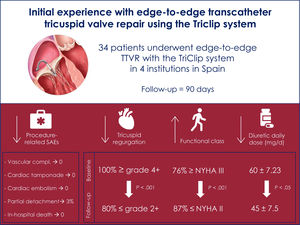
ResultsWe included 34 patients. Most of them reported a previous history of atrial fibrillation (91%) and only 1 had a pacemaker lead. The primary endpoint (TR reduction of at least 1 grade at discharge) was met in all patients. Most of the patients required 1 (47%) or 2 clips (44%) with a clear predominance of XT (87%) over NT (13%). The location of the first clip was anteroseptal in >90% of the patients. Only 1 patient had a partial detachment, which was stabilized with additional clips in the same procedure. At discharge, TR severity was≤2 in 91% of patients. At 3 months, mortality was nil. Overall, 88% of patients were in New York Heart Association functional class≤2 and 80% had residual TR≤2.
ConclusionsEdge-to-edge TTVR seemed to be effective and safe with a sustained TR reduction at 3 months. Further studies will be needed to confirm our findings.
Keywords
Tricuspid regurgitation (TR) is a frequent heart valvular disease that is directly linked to increased morbidity and mortality.1,2 Generally, the insidious and fluctuating symptoms of TR translate into substantial delays in applying invasive corrective strategies.3 These delays usually impact on higher comorbidities at the time of correction and therefore high mortality rates of up to 10% with isolated surgical interventions.4,5 Percutaneous tricuspid interventions are progressively offering alternatives to conventional surgery in high-risk patients.6,7 Among them, edge-to-edge transcatheter tricuspid valve repair (TTVR) represents the strategy with the highest penetration worldwide.7 Compassionate use of MitraClip devices (Abbott Medical, United States) in a tricuspid position was initially proposed with promising results.7,8 Nonetheless, the difficulties in achieving proper catheter height or optimal coaxiality represented major limitations for the conventional mitral system.9 TRILUMINATE Pivotal was the first trial assessing the performance of a dedicated edge-to-edge TTVR system called TriClip (Abbott Medical).10,11 Despite the use of only NT devices (short arms), TriClip showed a high procedural success rate with a very low incidence of complications. At 1 year, TR was reduced to moderate or less in 71% of participants and most patients (83%) were in New York Heart Association (NYHA) functional class ≤ 2.11 The novel TriClip system became commercially available in Europe in June 2020 with 2 sizes: XT and NT. The present article describes the initial experience with the system in Spain.
METHODSStudy design and populationThis was a multicenter and retrospective analysis collecting individual data from 4 Spanish centers with initial access to the limited release of the TriClip system on the market for edge-to-edge TTVR in patients with symptomatic TR. Demographics, baseline and procedural characteristics and clinical/echocardiographic outcomes at follow-up were prospectively collected in a dedicated, shared, and prospective database at each participating center. Patients were evaluated by a Heart Team that consisted of interventional cardiologists, heart failure specialists, expert imaging cardiologists, and heart surgeons. All procedures were performed under general anesthesia and guided by transesophageal echocardiogram and fluoroscopy. Periprocedural complications were noted during the index hospitalization. The study was conducted in accordance with the institutional ethics committee of each participating center, and all patients provided signed informed consent for the procedure.
Endpoints and definitionsStandardized definitions of all patient-related variables, clinical diagnoses, and in-hospital complications and outcomes were used. We assessed TR using standard 2-dimensional color Doppler methods and graded using a 5-class grading scheme: mild, moderate, severe, massive, and torrential.12 Coaptation gaps were assessed in the short-axis transgastric view. Implant success was defined as successful delivery and deployment of at least 1 clip with achievement of leaflet approximation and retrieval of the delivery catheter. The primary endpoint was a TR reduction of at least 1 grade assessed by transthoracic echocardiography at discharge. Procedural and in-hospital major adverse events included death, cardiac tamponade, emergent surgery, vascular complications, major bleeding, stroke, and myocardial infarction. Clinical and echocardiographic follow-up was performed at 3 months. Clinical status was assessed by New York Heart Association (NYHA) functional class.
Statistical analysisCategorical variables are presented as frequencies (percentages), assessing the differences by the chi-square test (or Fisher test when necessary). Continuous variables are presented as the mean±standard deviation or as the median [interquartile range]. The Kolmogorov-Smirnov test and Shapiro-Wilk test were applied to ensure normal distribution. Paired t tests were used to analyze changes in continuous variables between baseline and follow-up visits and Friedman tests were used for paired nominal data. Follow-up was considered to terminate at the date of the last follow-up. Analyses were performed using STATA software (V 14.0, StataCorp LP, College Station, United States).
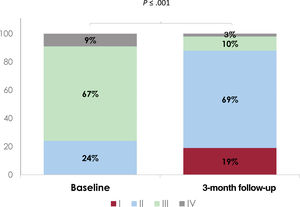
RESULTSBetween June 2020 and March 2021, a total of 34 patients underwent edge-to-edge TTVR with the TriClip System. Baseline characteristics are shown in table 1. Noteworthy, most of the patients referred previous history of atrial fibrillation and only 1 had a permanent pacemaker lead. Echocardiography revealed severe (47%) and massive (44%) TR and functional TR as the underlying mechanism (79%) in most patients (table 2). In addition, most of the TR jets were central (91%) and located at the anteroseptal line of coaptation of the valve (59%). Two patients had more than moderate MR: 1 patient underwent concomitant transcatheter mitral valve repair and in the other patient, the mitral valve was not suitable for transcatheter mitral valve repair. None of the patients presented pulmonary hypertension.
Baseline clinical characteristics
| Variable | n=34 |
|---|---|
| Age, y | 75.5 [69-79] |
| Sex, female | 25 (74) |
| BMI | 26.8±4.86 |
| Hypertension | 19 (56) |
| Diabetes mellitus | 7 (20) |
| Atrial fibrillation | 31 (91) |
| COPD | 5 (15) |
| CAD | 2 (6) |
| Stroke/TIA | 6 (18) |
| CKD | 14 (41) |
| eGFR (mL/min) | 57.8±21.7 |
| PPM/ICD lead | 1 (3) |
| Previous valvular surgery | |
| Mitral | 9 (27) |
| Tricuspid | 1 (3) [DeVega Annuloplasty] |
| Previous Mitraclip | 2 (6) |
| EuroSCORE II | 4.04 [2.31-5.68] |
| STS | 3.73 [2.76-5.44] |
| NYHA | |
| I | 0 |
| II | 8 (24) |
| III | 22 (67) |
| IV | 3 (9) |
| Peripheral edema | 29 (85) |
| Ascites | 15 (44) |
| Medical treatment | |
| Betablockers | 16 (47) |
| ACEI / ARA-II | 12 (34) |
| Aldosterone antagonists | 19 (56) |
| Sacubitril/valsartan | 0 |
| VKA | 25 (74) |
| NOAC | 4 (12) |
| Nitrates | 1 (3) |
| Hidralazine | 1 (3) |
| Furosemide | 31 (92) |
| Average daily dose (mg/d) | 60±7.23 |
| Tiazide | 10 (29) |
ACEI, angiotensin-converting enzyme inhibitors, ARA, angiotensin receptor antagonist; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ICD, implantable cardiac defibrillator; NOAC, novel oral anticoagulation; NYHA, New York Heart Association functional class; PPM, permanent pacemaker; STS, Society of Thoracic Surgeons; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
Baseline echocardiographic and right heart catheterization variables
| Echocardiographic variables (n=34) | |
| LVEF | 57.5 [55-61] |
| LVEDV (mL/m2) | 72 [53-80] |
| LVESV (mL/m2) | 29 [22.8-38] |
| LA volume (mL/m2) | 87 [52.6-114] |
| MR severity | |
| No | 8 (24) |
| Mild | 20 (61) |
| Moderate | 3 (9) |
| Moderate-severe | 1 (3) |
| Severe | 1 (3) |
| TR severity | |
| No | 0 |
| Mild | 0 |
| Moderate | 0 |
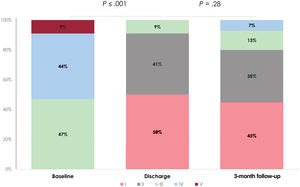
| Severe | 16 (47) |
| Massive | 15 (44) |
| Torrential | 3 (9) |
| TR mechanism | |
| Functional | 27 (79) |
| Degenerative | 1 (3) |
| Mixed | 6 (18) |
| TR lead induced | 1 (3) |
| Vena contracta (cm) | 1.32 [0.9-12] |
| Central location | 31 (91) |
| Max gap (mm) | 4.98±2.64 |
| Main jet location | |
| Anteroseptal | 20 (59) |
| Posteroseptal | 5 (15) |
| Anteroposterior | 9 (27) |
| RV fractional area change | 40 [35-47] |
| RVED area (cm2) | 21.6 [19-28] |
| RA area (cm2) | 28 [22.7-35] |
| TAPSE (cm) | 18 [15-20] |
| sPAP by echo (mmHg) | 40 [35-47] |
| IVC collapse> 50% | 6 (19) |
| IVC max diamater (mm) | 23 (20-27) |
| Right heart catheterization (n=16) | |
| sPAP (mmHg) | 29 [21-34.5] |
| RV peak (mmHg) | 27 [23-35.5] |
| RA peak (mmHg) | 8 [5.5-15.5] |
IVC, inferior vena cava; LA, left atrium; LVEDV, left ventricle end-diastolic volume; LVEF, left ventricle ejection fraction; LVESV, left ventricle end-systolic volume; MR, mitral regurgitation; PAP, pulmonary artery pressure; RA, right atrium; RV, right ventricle; RVED, right ventricle end-disastolic; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
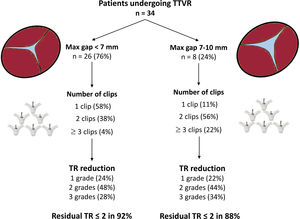
Procedural information is available in table 3. Acute reduction of TR ≥ 1 grade was achieved in all patients. Most of patients required 1 (47%) or 2 clips (44%) with a clear predominance of XT (87%) over NT (13%). Location of the first clip was anteroseptal in> 90% of cases. Only 1 patient presented a partial detachment that could be stabilized with 1 additional clip. Tricuspid regurgitation reduction was similar among patients with <7 mm or 7 to 10 mm leaflet gaps (figure 1). The primary endpoint (TR reduction of at least 1 grade at discharge) was met in all patients. Post-procedural and predischarge TR severity was ≤ grade 2 in 97% and 91% of patients respectively. The median time of hospital stay was 2 [1-3] days.
Procedural data
| Variable | n=34 |
|---|---|
| Number of clips per patient | |
| 1 | 16 (47) |
| 2 | 15 (44) |
| 3 | 2 (6) |
| > 3 | 1 (3) |
| Type of clip NT/XT | |
| NT | 6 (13) |
| XT | 40 (87) |
| Location first clip | |
| Anteroseptal | 31 (91) |
| Posteroseptal | 3 (9) |
| Anteroposterior | 0 |
| Location second clip | |
| Anteroseptal | 14 (74) |
| Posteroseptal | 5 (26) |
| Anteroposterior | 0 |
| Final TV mean gradient (mmHg) | 1.4 [1-2] |
| Procedural time (min) | 130 [100-173] |
| Fluro time (min) | 30 [21.75-43.6] |
| Final in-lab TR severity | |
| No | 0 |
| Mild | 23 (68) |
| Moderate | 10 (29) |
| Severe | 1 (3) |
| Massive | 0 |
| Torrential | 0 |
| Technical complications | |
| No | 33 (97) |
| Partial dettachment | 1 (3) |
| Total dettachment | 0 |
| Leaflet perforation | 0 |
| Chordae rupture | 0 |
| Clinical complications | |
| No | 32 (94) |
| Death | 0 |
| Cardiac tamponade | 0 |
| Emergent surgery | 0 |
| Vascular complications | 0 |
| Major bleeding | 0 |
| Stroke | 0 |
| Myocardial infarction | 0 |
| Admission days | 2 [1-3] |
| Pre-discharge TR severity | |
| No | 0 |
| Mild | 17 (50) |
| Moderate | 14 (41) |
| Severe | 3 (9) |
| Massive | 0 |
| Torrential | 0 |
| Antithrombotic strategy at discharge | |
| None | 1 (3) |
| SAPT | 3 (9) |
| DAPT | 1 (3) |
| VKA | 23 (68) |
| NOAC | 6 (17) |
DAPT, dual antiplatelet therapy; NOAC, novel oral anticoagulation; SAPT, single antiplatelet therapy; TR, tricuspid regurgitation; TV, tricuspid valve; VKA, vitamin K antagonist.
Data are expressed as no. (%) or median [interquartile range].
Clinical and echocardiographic follow-up was available in all patients (table 4). At 3 months, no mortality was reported. Overall, 88% of patients were in NYHA functional class ≤ 2 and only 3 (9%) participants required admission for heart failure (figure 2). One patient (3%) with residual severe TR underwent a repeated procedure 1 month after the first one. More than half of patients reduced the dose of diuretic at 3 months follow-up. Residual TR was ≤ grade 2 in 80% of patients (figure 3). Importantly, echocardiographic parameters namely right ventricle end-diastolic (RVED) area, TR vena contracta and inferior vena cava (IVC) maximum diameter improved compared with baseline (table 5).
Clinical and echocardiography follow-up at 3 months
| Clinical follow-up at 3 months | |
| Mortality | 0 |
| CV mortality | 0 |
| Admissions for HF | 3 (10) |
| NYHA | |
| I | 6 (19) |
| II | 22 (69) |
| III | 3 (10) |
| IV | 1 (3) |
| Peripheral edema | 10 (29) |
| Ascites | 3 (10) |
| Diuretic dose modification | |
| No | 13 (41) |
| Yes, reduce | 17 (53) |
| Yes, increase | 2 (6) |
| Furosemide average daily dose (mg/d) | 45±7.5 |
| Transthoracic echocardiography at 3 months | |
| TR severity | |
| No | 0 |
| Mild | 14 (45) |
| Moderate | 11 (35) |
| Severe | 4 (13) |
| Massive | 2 (7) |
| Torrential | 0 |
| RA area (cm2) | 28.1 [22-36] |
| RVED_area (cm2) | 18.8 [16.8-24.8] |
| RV fractional area change | 38.5 [33-47] |
| TAPSE (cm) | 1.8 [1.4-1.9] |
| Vena contracta (cm) | 0.6 [0.3-0.9] |
| IVC collapse> 50% | 13 [54] |
| IVC max diamater (mm) | 18 [16-23] |
CV, cardiovascular; HF: heart failure; IVC, inferior vena cava; NYHA, New York Heart Association; TR, tricuspid regurgitation; RA, right atrium; RV, right ventricle; RVED, right ventricle end-diastolic; TAPSE, tricuspid annular plane systolic excursion.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
Echocardiographic variables at baseline and 3-month follow-up
| Variable | Baseline | 3-month follow-up | Change | P |
|---|---|---|---|---|
| RA area | 28 [22.7-35] | 28.1 [22-36] | –0.7 [–6-4] | .31 |
| RVED_area | 21.6 [19-28] | 18.8 [16.8-24.8] | –2.1 [–4.9-0] | .021 |
| RV fractional area change | 40 [35-47] | 38.5 [33-47] | 0 [–8-6] | .818 |
| TAPSE | 18 [15-20] | 18 [15-20] | –0.3 [–6-0.4] | .10 |
| Vena contracta | 1.32 [0.9-12] | 0.6 [0.3-0.9] | –0.6 [–0.87-(–0.27)] | .018 |
| IVC max diamater | 23 [20-27] | 18 [16-23] | –4 [–5.5-(–1)] | .013 |
IVC, inferior vena cava; RA, right atrium; RV, right ventricle; RVED, right ventricle end-disastolic; TAPSE, tricuspid annular plane systolic excursion.
The main findings of the present manuscript were the following: Edge-to-edge TTVR with the TriClip system was a) Feasible and safe with a high procedural success rate and a low incidence of complications; b) Effective as depicted by a TR reduction of at least 1 grade in all patients and the presence of residual TR ≤ 2 in 91% and 80% at discharge and at 3 months, respectively; c) Clinically relevant as most patients were in NYHA functional class ≤ 2 (88%) at 3 months; d) Translated into improvement of echocardiographic parameters namely RVED area, TR vena contracta and IVC max diameter at 3 months; and e) Associated with a major use of XT (87%) compared with NT devices (13%).
Among other results, the observed TR reduction with the absence of major clinical complications is probably the most important finding of the study. Availability of a TTVR technique that is highly effective for TR reduction but at the same time highly safe, represents a major advantage for the device/technique. As shown in table 3, no patient experienced any relevant clinical complication that could be related to the procedure. Prior to the development of the TriClip system, TTVR was mainly performed using nonspecific tricuspid edge-to-edge devices such as the MitraClip system. TriClip system is based on the same technology as MitraClip, but the new guiding catheter has been specifically designed to approach the tricuspid valve, adapting to the right side of the heart, and thus increasing the safety and efficacy of the procedure.6 In fact, the largest registry of patients using edge-to-edge TTVR before the arrival of TriClip showed an acute successful tricuspid repair in 83% of patients compared with our 100% acutely or the 87% at 30 days in the TRILUMINATE trial.1–13
On the other hand, the observed results were even better than those observed in the TRILUMINATE trial, in which despite that 87% of patients experienced at least 1 grade of TR improvement, only 60% of them presented TR ≤ 2 at 30 days.10,11 Interestingly, the reported 60% at 30 days improved to 70% at 1 year as a result of the inverse remodeling of the right heart chambers.11 Our series showed TR reduction of at least 1 grade in all patients and residual TR ≤ 2 in 80% of patients at 3 months. The improved results may respond to several factors but the increasing experience of operators and availability of not only NT (short arms) but also XT (long arms) devices are probably the most important factors that might explain these promising results. In fact, in the present series, the XT system was used in 87% of the implanted clips. Availability of the XT clip likely played a very important role on procedural results by facilitating the grasping process in any anatomy, but especially in valves with large coaptation gaps. The NT clip was generally left for more commissural grasping in which operators could anticipate a higher risk of entrapment with the sub-valvular apparatus. Most of patients (91%) presented central jets and many were treated with just 1 clip (47%). Similarly, to previous reported experiences,11,13,14 the first clip was implanted anteroseptal in 91% of them. A second clip was necessary in 44%. This second clip was also implanted anteroseptal in 75% and postero-septal in 26% of patients. Three clips were necessary in 2 patients (6%) and 1 patient (3%) required 5 clips. The patient with 5 clips presented a partial detachment that could be detected during the procedure and fixed with additional clips in the same intervention. At 3 months, no other cases of partial or total detachment were detected. In contrast to previous publications,13 the presence of a gap ≥ 7 mm did not seem to limit the effectiveness of the therapy as the grade of TR reduction was similar among patients with <7 mm and 7 to 10 mm gaps. Again, the present finding might be explained by the increasing experience and the current XT device availability. Moreover, as shown in figure 1, patients with gaps≥7 mm required a higher number of clips. In any case, it is important to acknowledge that the maximum treated gap in this series was 10mm so the results with gaps beyond 10 mm are uncertain.
The present series did not collect any case with the last device generation: TriClip G4. The G4 system is already in the market and provides additional features that may improve the results of the procedure. TriClip G4, like MitraClip G4, comes in 4 different sizes: the regular NT and XT but also the wide versions: NTW and XTW.15 TriClip G4 also allows independent grasping from the gripper line. Independent grasping is a novel feature that might be very useful in the tricuspid field for 2 reasons. First, it might help to treat large coaptation gaps, and, second, it may allow to improve visualization of the grasping process in cases of challenging transesophageal echocardiogram window as the echocardiographer may focus individually on one or another leaflet. Additionally, for suboptimal leaflet grasping, the independent gripper line may help to optimize a one-side leaflet insertion without losing the other-side leaflet.
Despite the limited follow-up, most of patients experienced clinical improvement as depicted by a NYHA functional class ≤ 2 at 3 months in 87% of patients (figure 4). The present results are in agreement with the ones observed in the TRILUMINATE trial in which 82% and 83% of patients were in NYHA class ≤ 2 at 30 days and 1 year, respectively.11 On the other hand, only 3 patients experienced a new hospitalization for heart failure at 3-month follow-up. A total of 2 of these patients had torrential TR before TTVR that was reduced to severe after TTVR. In TRILUMINATE trial, the persistent of severe or more TR after TTVR was associated with increased mortality and heart failure hospitalizations at 1 year, with nearly a 3-fold increase in participants with severe or more TR compared to those with moderate or less TR after TTVR11 (24.5% vs 8.8%, respectively). This fact emphasizes the effects of TR reduction on clinical outcomes in patients undergoing TTVR: the greater the reduction in TR, the greater the clinical benefits. In our series, in more than half of patients (53%) the diuretic dosage could be reduced and only in 6%, diuretic scalation was required. Modification of the diuretic dosage is currently a matter of controversy. Some physicians advocate for maintaining the diuretic dosage for 3 months to promote the reverse remodeling of the right chambers by keeping a residual TR as low as possible. In fact, reverse remodeling of the right chambers is probably one of the reasons that explain why both clinical and echocardiographic benefits seem to further progress at follow-up.10,11 As shown in table 5 and despite the limited number of patients, the RVED area decreased at 3 months indicating some degree of reverse remodeling. Reverse remodeling seems to occur early after the procedure.10 In the TRILUMINATE trial, early (30 days) and sustained (1 year) reductions of the RA and RV volumes could be observed.10,11 A more progressive improvement of the tricuspid annular plane systolic excursion (TAPSE) within the first year could also be seen.11 In our study, the absence of significant differences in the TAPSE may respond to the limited follow-up and the fact that TAPSE was high (1.8 cm) at baseline, indicating the relatively preserved status of the RV function at baseline in most of patients.
The present study has several limitations. This was a small observational registry which implies an inherent selection bias. Moreover, it is difficult to capture and control all potential confounders when using a registry. In addition, the sample size may lack power to detect predictors of improvement in NYHA class function, echocardiographic variables, or predictor of procedural failure. Both clinical and echocardiographic data were self-reported by the centers so no independent adjudication for clinical events or core-lab for echo analysis. Our cohort had a lower Euroscore II compared to TRILUMINATE cohort. This could be explained by the fact that the main contraindication for cardiac surgery in our cohort was the presence of frailty, a clinical characteristic that has been associated with a worse prognosis after cardiac surgery16 and which is not assessed in Euroscore II. Right heart catheterization is the gold standard for the assessment of the severity of pulmonary hypertension. In our cohort, only 16 patients underwent right heart catheterization prior to TTVR. The main reason for not performing a right hear catheterization in these patients was the absence of left heart disease and indirect echocardiographic signs of pulmonary hypertension. Conducting health-related quality of life questionnaires and 6-minute walk tests before and after TTVR could help us better understand the impact of this promising therapy.
CONCLUSIONSThe present manuscript provides the initial experience with the use of the TriClip system for an edge-to-edge TTVR after a limited release in experienced centers. The device seemed to be effective and safe with promising results in terms of TR reduction not only acutely but also at 3 months follow-up. Further studies with larger sample and longer follow-up will be needed to confirm our findings.
- -
Tricuspid regurgitation has received increasing interest in recent years, mainly because of its association with an increased risk of morbidity and mortality.
- -
Recently, transcatheter tricuspid valve repair techniques have emerged as an alternative for the treatment of severe tricuspid regurgitation in patients ineligible for surgery.
- -
Edge-to-edge transcatheter tricuspid valve repair with the TriClip system is the strategy with the highest penetration worldwide
- -
The present study describes the initial experience with the use of the TriClip system for edge-to-edge transcatheter tricuspid valve repair after limited release in experienced centers.
- -
Edge-to-edge transcatheter tricuspid valve repair with the TriClip system was feasible and safe with a high procedural success rate and a low incidence of complications.
- -
The presence of residual tricuspid regurgitation ≤ 2 was achieved in 91% and 80% of the cohort at discharge and at 3 months, respectively.
No funding.
AUTHORS’ CONTRIBUTIONSX. Freixa, D. Arzamendi, R. Estévez-Loureiro and J. Goicolea conceived and designed the analysis. M. del Trigo, P. Li, L. Sanchis and Manuel Barreiro collected the data. X. Freixa and P. L. Cepas-Guillén performed the analysis. A. Regueiro, J. A. Baz, L. Asmarats, F. Calvo, V. Moñivas, I. Meduiña and M. Sitges reviewed and edited the manuscript.
CONFLICTS OF INTERESTX. Freixa, L. Sanchis, D. Arzamendi, R. Estévez-Loureiro and M. Sitges have served as proctors for Abbott. R. Estévez-Loureiro indicates speaker fees from Abbott, Boston Edwards Lifesciences and P&F. L. Sanchis is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed.