In the last few years, there has been growing interest in the relationship between cancer and cardiovascular disease. The increase in life expectancy in both diseases has led to their frequent coexistence in the same patient, which can lead to adverse drug reactions that increase patient risk. This is especially relevant in the case of atherosclerosis, which seems to share a common pathophysiological substrate with cancer. In this review, we analyze these common risk factors, and specifically analyze the relationship between different cancer treatments with the risk of coronary or cerebrovascular disease, as well as the current scientific evidence on the possible relationship between antiplatelet therapy and cancer risk. We also review the incidence and prognosis of cancer in patients with atherosclerosis and vice versa, based on the information reported in the most recently published studies in the field of cardio-oncology.
Keywords
Cardiovascular disease (CVD) and neoplasms are the first and second leading causes of death, respectively, worldwide.1 However, the downward trend seen in CVD incidence and mortality in recent decades has not been observed in neoplasms, which have exhibited an increase in incidence and no relevant reductions in mortality in most cases.2 In Europe alone, in 2012 there were more than 3.5 million cases of de novo cancer and nearly 2 million deaths due to these diseases.3 National estimates forecast a rise of more than 30% in the incidence of cancer in Spain compared with past decades, mainly due to an aging population and changes in risk factors.4
In recent years, there is stronger interest in the incidence and mortality of CVD in patients treated for neoplasms, as up to 30% of deaths among cancer patients may have a cardiovascular cause.5 Furthermore, the progressive drop in mortality among patients with acute coronary syndrome (ACS) or stable chronic ischemic heart disease has also increased long-term survival in these patients, who are at greater risk of de novo tumors or recurrence of previous diseases, thus posing a major challenge for clinical care and affecting the prognosis of these patients.
COMMON RISK FACTORS FOR CANCER AND ATHEROSCLEROSISBecause cancer and atherosclerosis share risk factors, concomitant ischemic heart disease and neoplasms in the same person are not rare, and 4% to 10% of patients with ACS or chronic ischemic heart disease have a history of cancer.6,7 The RECALCAR (REcursos y CALidad en CARdiología [Resources and Quality in Cardiology] registry describes a prevalence of 2.77% for malignant tumors in patients hospitalized for ACS, associated with higher in-hospital mortality (odds ratio=2.26; 95% confidence interval [95%CI], 1.99-2.55).6 A prospective study of all patients hospitalized for ACS in a single Spanish center between 2009 and 2016 observed that neoplasms were present at admission in 3.4% (95%CI, 2.7-4.4) of these patients and that the most common sites were the colon, bladder, lung, and prostate.8 The median time between neoplasm diagnosis and ACS was 5.5 years, and most patients had undergone cancer-specific surgery (74.2%) or chemotherapy (46.8%). Only 41.9% of patients were considered disease-free when hospitalized for ACS, which had major implications for in-hospital treatment and long-term prognosis.
Age, sedentary lifestyle, smoking, and a fat- and carbohydrate-rich diet are established risk factors for CVD, but also for neoplasms.9 The latest data from REDECAN (Red Española de Registros de Cáncer [Spanish Network of Cancer Registries]) in Spain show that the most common cancers are prostate, colon, lung, and bladder neoplasms in men and breast, colon, uterus, and lung in women,10 consistent with large registries in Europe3 and the United States.2 Smoking is the risk factor most strongly associated with ischemic heart disease and neoplasms and, in fact, is considered the leading cause of avoidable mortality due to its impact on the first 2 causes of death worldwide.11 An analysis of the Spanish health survey of 2011 to 2012 and the vital statistics of the National Institute of Statistics showed that the total prevalence of smoking in Spain in 2012 was 23.6%, and it was estimated that 125 men and 40 women died each day due to causes attributable to tobacco use, accounting for over 60 000 deaths in 1 year.12 The main causes of smoking-related death were tracheal, bronchial, and lung cancer in men and heart disease in women. Smoking is also a major risk factor in the incidence of ACS, particularly in young people.12 Because mortality among young patients with ACS is very low, most patients survive for a considerably long period and are susceptible to cancers over time. In fact, recent data show that patients who were smokers or exsmokers at the time of ACS have a 3-fold risk of neoplasm after ACS.
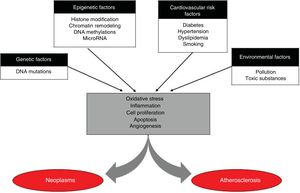
Diabetes mellitus is one of the cardiovascular risk factors that most increases the risk of death due to ischemic heart disease. Extensive follow-up of more than 400 000 people with diabetes in Sweden, compared with more than 2 million controls, showed that patients with diabetes had higher mortality due to CVD, but also due to cancer.13 Furthermore, that study showed that metabolic control of diabetes, assessed by blood glucose and glycohemoglobin concentration, was clearly associated with cardiovascular and noncardiovascular mortality, and revealed that hyperglycemia played a key role in activating other pathophysiologic pathways. The stronger presence of cardiovascular risk factors also produced a linear increase in serum inflammation markers, such as C-reactive protein, shown to be an independent marker of the risk of ischemic heart disease incidence and mortality.14 Of these, diabetes mellitus by itself has the highest levels of C-reactive protein and is also the risk factor most strongly associated with ischemic heart disease.14 Although the etiology of cancer varies considerably, the underlying pathophysiology for almost neoplasms is a chronic inflammatory condition and a lack of resolution of proinflammatory stimuli that alter the immune system; this phenomenon is very similar to macrophage activation in the artery wall due to low-density-lipoprotein accumulation.15 Therefore, chronic activation of the immune system and the inflammatory state underlie the pathophysiology of both atherosclerosis and neoplasms, which would explain many of the CANTOS16 findings. That study showed that canakinumab, a monoclonal antibody that blocks the action of interleukin 1ß, found in the pathway where interleukin 6 and C-reactive protein act, reduced the incidence of major cardiovascular complications and cancer in persons with a history of acute myocardial infarction (AMI) and elevated C-reactive protein (> 2mg/L).16 The decrease in the incidence of the primary endpoint (AMI, stroke, or cardiovascular death) was related to the canakinumab dose received, which was also very closely associated with decreases in C-reactive protein and interleukin 6. A subsequent subanalysis showed that the incidence of lung cancer and cancer death was noticeably lower in patients who received canakinumab than in those who received placebo, and that this reduction was dose-dependent.17 Because canakinumab did not affect serum glucose or lipid concentration,18 nor did it have any direct impact on oncogenesis, it appears to be clearly proven that inflammation plays a critical role in both atherosclerosis and the appearance of neoplasms.
Apart from inflammation, which also seems be a possible target for common treatment, atherothrombotic disease and neoplasms share many pathophysiologic pathways (Figure 1). Cell proliferation and apoptosis are 2 of the key mechanisms of tissue homeostasis. A change in homeostatic balance often leads to neoplasms, while also being related to the development and destabilization of coronary atheromatous plaques.19 Another of the characteristic mechanisms of neoplasms is neoangiogenesis, which has also been linked to their capacity for local and remote invasion because cell proliferation requires oxygen and nutrients. Similarly, the formation of neovessels, known as vasa vasorum, inside the plaque creates one of the main entry points for lipoproteins, erythrocytes, and inflammatory cells in plaques. The role of these neovessels is 2-fold, as they can be a recovery mechanism when lipoprotein concentrations drop due to intensive lipid-lowering treatment; however, it has also been reported that a large part of these neovessels are highly dysfunctional, promote particle passage to the plaque, and produce destabilization.19 Another common major pathophysiologic mechanism between cancer and atherosclerosis consists of RNA microparticles, which are noncoding fragments of messenger RNA that regulate the posttranscriptional expression of genes. These microparticles are released as a cellular response to any aggression and have been clearly related to oxidative stress and inflammation; hence, it is not surprising that various molecules related to both atherosclerosis and various neoplasms have been identified.20
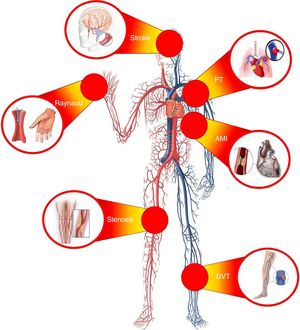
ATHEROSCLEROTIC DISEASE IN PATIENTS WITH CANCER: INCIDENCE AND PROGNOSISOngoing advances in the treatment of neoplastic processes have significantly increased the survival of cancer patients and, consequently, have affected the appearance of high rates of comorbidities and medical complications associated with or favored by cancer. This means that CVD is one of the main causes of morbidity and mortality in patients with malignant neoplasms (Figure 2). For instance, women with an early diagnosis of breast cancer are more likely to die from CVD than from the cancer itself.21
Compared with patients not diagnosed with cancer, adults who experience a neoplastic process have a significant increase in CVD risk. A recent study has shown that patients newly diagnosed with cancer had an almost 3-fold risk (hazard ratio [HR]=2.9; 95%CI, 2.8-3.1) for AMI compared with that of the control population,22 and that the increase in risk was proportional to cancer stage (significantly higher in patients with greater tumor burden or disease spread). That study specifically covered the period in which this excess cardiovascular risk is higher (limited to the first year after the cancer diagnosis), which may be particularly relevant when considering possible primary prevention strategies, such as antithrombotic therapy or statins, in these stages.
Although cancer can cause atherosclerosis through different mechanisms, the most common are sequelae of antitumor drugs and radiotherapy (RT). However, many patients with cancer and CVD have a common substrate—apart from RT and chemotherapy—that links the 2 conditions. For instance, patients with cancer are often found to have metabolic and vascular abnormalities, among them, abdominal obesity, altered glucose metabolism, lipoprotein abnormalities, and hypertension.23
Before discussing the increased cardiovascular risk associated with RT and chemotherapy, it is interesting to mention the cardiovascular risk of patients with hematologic neoplasms who have undergone hematopoietic cell transplantation, mainly due to graft-versus-host disease (endothelial lesion), increase in cardiovascular risk factors due to immunosuppressive treatment (accelerated atherosclerosis), and sedentary lifestyle (proatherosclerotic habits). A study by Chow et al.24 in more than 1000 patients who had received hematopoietic cell transplantation found that the risk of a coronary event was more than 3-fold that of the control group, consistent with previous studies reporting that up to 1 of every 5 patients who had received allogenic hematopoietic transplantation had been diagnosed with CVD within 20 years, an onset of ischemic heart disease about 10 to 15 years earlier than in the control population.
Radiotherapy and atherosclerotic riskChest RT—used mainly to treat breast cancer and Hodgkin's lymphoma—is associated with a higher risk of ischemic heart disease, whereas cerebral RT—used in primary tumors of the central nervous system—has been associated with an increased risk of cerebrovascular disease.25 In fact, chest RT has been reported to raise the relative risk of AMI or sudden death 5- to 10-fold, whereas cerebral RT increases the risk of stroke 20-fold.26 The pathophysiologic basis is injury to the vascular wall: RT induces endothelial dysfunction that increases capillary permeability and activates inflammation, which leads to intimal proliferation, collagen formation and deposits, and fibrosis, favoring the formation of atherosclerosis plaques. Although there are no reports of an apparent threshold below which there is no risk of cancer, there is a direct association between RT dose and the risk of cancer. A study in women with breast adenocarcinoma treated with RT observed that, for every 7Gy of radiation received, the risk of a coronary event increased 7.4%27; the risk of ischemic heart disease was particularly high with RT doses ≥ 10Gy (increase of 116%; 95%CI, 59-195). That risk becomes evident after 5 years and persists up to 30 years after RT. In the case of chest RT after Hodgkin's lymphoma, it was observed that up to 10% of treated patients had experienced a cardiovascular event within 11 years afterward.28 Last, the study by Heidenreich et al. analyzed 300 asymptomatic patients who had received RT for Hodgkin's lymphoma 15 years earlier, observing that 1 of every 5 had an abnormal echocardiogram, 1 of every 7 had a perfusion defect on stress echocardiography, and 1 of every 14 had proven arterial disease with coronary stenosis> 50%.29
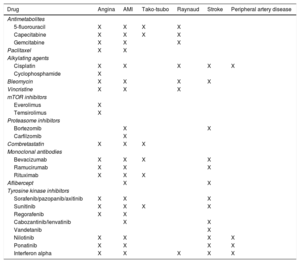
Chemotherapy and atherosclerotic diseaseA number of chemotherapy drugs have been associated with an increased incidence of atherosclerotic CVD (Table 1), although the cumulative incidence of ischemic heart disease is usually fewer than 5 cases per 100 patients a year in those treated with chemotherapy.30 Most notably, the chemotherapy drugs with the highest atherosclerotic risk are the antimetabolites, antimicrotubule agents, and tyrosine kinase inhibitors. In addition, in the case of fluorouracil, the vasospasm rate is around 40% to 70% of patients, in the various series, and the event occurred within a few hours or days of treatment.31 This drug should not be administered over more than 3 hours or in combination with cisplatin. Tyrosine kinase inhibitors, particularly nilotinib, have been associated with accelerated arteriosclerosis, particularly of peripheral manifestation, reported in approximately 10% to 25% of patients,32 a risk not extrapolated to imatinib. A higher risk of arterial thrombosis has also been reported.
Chemotherapy agents associated with cardiovascular diseases
| Drug | Angina | AMI | Tako-tsubo | Raynaud | Stroke | Peripheral artery disease |
|---|---|---|---|---|---|---|
| Antimetabolites | ||||||
| 5-fluorouracil | X | X | X | X | ||
| Capecitabine | X | X | X | X | ||
| Gemcitabine | X | X | X | |||
| Paclitaxel | X | X | ||||
| Alkylating agents | ||||||
| Cisplatin | X | X | X | X | X | |
| Cyclophosphamide | X | |||||
| Bleomycin | X | X | X | X | ||
| Vincristine | X | X | X | |||
| mTOR inhibitors | ||||||
| Everolimus | X | |||||
| Temsirolimus | X | |||||
| Proteasome inhibitors | ||||||
| Bortezomib | X | X | ||||
| Carfilzomib | X | |||||
| Combretastatin | X | X | X | |||
| Monoclonal antibodies | ||||||
| Bevacizumab | X | X | X | X | ||
| Ramucirumab | X | X | X | |||
| Rituximab | X | X | X | |||
| Aflibercept | X | X | ||||
| Tyrosine kinase inhibitors | ||||||
| Sorafenib/pazopanib/axitinib | X | X | X | |||
| Sunitinib | X | X | X | X | ||
| Regorafenib | X | X | ||||
| Cabozantinib/lenvatinib | X | X | ||||
| Vandetanib | X | |||||
| Nilotinib | X | X | X | X | ||
| Ponatinib | X | X | X | X | ||
| Interferon alpha | X | X | X | X | X | |
AMI, acute myocardial infarction; mTOR, mammalian target of rapamycin.
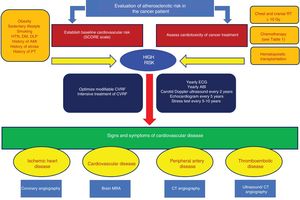
There are no firm recommendations on the need to perform regular screening tests for subclinical atherosclerosis in these patients, although several scientific societies have issued consensus documents4,31,33 (Figure 3). Patients who have received chest RT, chemotherapy with high-risk drugs, or hematopoietic cell transplants should be considered at high cardiovascular risk. These patients should be advised to maintain healthy lifestyle habits and strict risk factor control, but there are no guidelines on the prophylactic use of antiplatelet agents. For patients with angina, the recommendation is to optimize antianginal therapy and to monitor triggers, such as anemia. In the case of persistent symptoms, patients should undergo ischemia diagnostic tests, and possibly revascularization to allow therapy to be tolerated, assessing the risks and benefits. The protocols for ACS are similar to those for noncancer patients, with an individualized approach taken to revascularization and antithrombotic regimens. However, patients with cancer and a history of AMI are often treated without adhering to the current scientific recommendations, which sometimes leads to a worse prognosis in terms of mortality.34 According to Mayo Clinic35 data, up to 1 of every 10 patients with AMI had a history of cancer, and these patients have higher noncardiovascular mortality rates, with no differences in short- and long-term cardiac mortality rates when they are treated according to the recommendations of clinical practice guidelines. It is important to consider that patients with cancer and an ACS have an increased risk of hemorrhagic events,36 whether in-hospital or during follow-up, making it essential for these patients to achieve a careful balance between ischemic risk and hemorrhagic risk when optimizing antiplatelet medication.37
Algorithm for assessment of atherosclerotic and thrombotic risks in patients with cancer. ABI, ankle-brachial index; AMI, acute myocardial infarction; CT, computed tomography; CVRF, cardiovascular risk factors; DLP, dyslipidemia; DM, diabetes mellitus; ECG, electrocardiogram; HTN, hypertension; MRA, magnetic resonance angiography; PT, pulmonary thromboembolism; RT, radiotherapy.
The incidence of neoplasms in patients with ACS has been insufficiently studied in contemporary series, despite abundant evidence to support a common pathophysiology. A recent cohort study with ACS patients showed that the incidence of neoplasms was 3.1% (95%CI, 2.4-4.0) during a mean follow-up of 33 months, and the most common sites were colon, lung, bladder, and pancreas.8 The median time to the appearance of new neoplasms was 25.0 [interquartile range, 12.0-56.0] months, and the factors associated with the appearance of cancer were age (HR=1.03; 95%CI, 1.01-1.06; P=.01) and smoking or history of smoking (HR=2.68; 95%CI, 1.11-6.49; P=.03).8 That study reported that patients who developed any type of neoplasm during follow-up had the highest mortality during this follow-up period (64.2%), followed by patients with neoplasms already known at the time of ACS (40.0%); additionally, more than half the deaths were directly attributed to neoplasms. However, higher cardiovascular mortality was not seen in patients with incident neoplasms, but was observed in patients with neoplasms present in ACS, a finding attributed by the authors to less aggressive ACS treatment, such as reduced use of drug-eluting stents or complete revascularization. These data show that patients with ACS who have or develop neoplasms have a worse and clearly different prognosis, revealing the need for very specific follow-up and treatment for these patients.
Several prospective and multicenter studies have found that the long-term mortality of patients with ACS or chronic ischemic heart disease is higher for noncardiovascular causes than cardiovascular causes8,34; however, the impact of neoplasms has been specifically investigated in only a few series. For example, the SYNTAX18 study in patients with stable chronic ischemic heart disease found a noncardiovascular mortality rate of 4.3% in patients who underwent percutaneous coronary intervention and 5.3% in patients treated by surgery, whereas cancer mortality was 2.2% and 2.4%, respectively.18
ANTIPLATELET THERAPY AND THE RISK OF CANCERThe effect of antiplatelet drugs on tumor growth and its prognostic implications in cancer patients is not a new topic, but the issue has become more relevant in scientific research in recent years. Some authors defend a complex causal association attributed to the direct action of certain antithrombotic drugs in cancer processes, inhibiting tumor growth and metastatic dissemination. However, most scientists advocate an incidental association, mediated by confounding factors, based on the idea that “cancer follows bleeding”, which lends credibility to the idea of an indirect association between antithrombotic medication and cancer. The evidence on the association between cancer and various antiplatelet drugs is described below.
AspirinAspirin has shown a favorable antitumor profile when used for many years. This is particularly relevant for the prevention of gastrointestinal cancers, with some controversy regarding its benefit in bladder and prostate cancer.38 There are a number of different mechanisms: direct inhibition of cyclooxygenase-2, prevention of carcinogenic agent activation by inhibiting sulfation of P-phenol sulfotransferase, flow reduction through decarboxylase ornithine thus promoting the antiproliferative activity of colon tumor cells, blockade of the inflammatory response in gene transcription, reduction of apoptosis by mitochondrial cytochrome release or positive regulation of apoptotic markers (Bcl-2 and Bax), and suppression of vascular endothelial growth factor.37
ClopidogrelCurrent evidence based on clinical trials and observational studies shows no firm association between clopidogrel and the risk of cancer, although there are many conflicting results. The CAPRIE39 and CHARISMA40 studies showed that, at 30 months, clopidogrel was not associated with a higher risk of cancer than placebo (in addition to aspirin). It is true that the CURE41 study on clopidogrel observed twice as many colorectal cancers as placebo, but this finding was not confirmed in the CAPRIE or CHARISMA study. It is also true that clopidogrel (vs placebo) was associated with more lung cancers in CURE (12 vs 7) and in CREDO42 (5 vs 0), but not in CAPRIE (72 vs 74) or CHARISMA (70 vs 63). In a large study with 183 912 patients and more than 20 000 cases of cancer, the combination of clopidogrel with aspirin was associated with even fewer cancers than aspirin as monotherapy (HR=0.92; 95%CI, 0.86-0.97).43 Recently, Kotronias et al.44 have published a meta-analysis with 282 084 patients, reporting no positive or negative association between clopidogrel and the appearance of cancer. This controversy was again raised when dual antiplatelet therapy was prolonged beyond the first year. The DAPT45 study found a significant increase (0.62% vs 0.28%; P=.02) of cancer deaths with prolonged dual antiplatelet therapy with clopidogrel or prasugrel. These findings are consistent with data from the KOREA46 registry, in which dual antiplatelet therapy for 30 months vs 12 months was associated with a significant increase in the incidence of cancer (4.15% vs 4.04%; HR=1.22; 95%CI, 1.06-1.41; P=.005). However, later studies have not observed a higher risk of cancer when dual antiplatelet therapy with clopidogrel lasted more than 1 year, as seen in the meta-analysis recently published by Emariah et al.,47 in which cancer mortality was similar, regardless of whether dual antiplatelet therapy was given> 12 months or ≤ 12 months (0.93% vs 0.99%; P=.59).
PrasugrelVarious studies in mice have shown a dose-dependent relationship between prasugrel and certain solid cancers, such as intestinal, lung, and liver tumors.37 The TRITON-TIMI 3848 study observed 174 cancers in the prasugrel group and 175 in the clopidogrel group. Once nonmelanoma skin cancers and brain tumors were excluded, 92 new solid cancers were recorded in the prasugrel group compared with 64 in the clopidogrel group; the relative risk of these solid cancers was 1.44 with prasugrel compared with clopidogrel (P=.024). This association of a higher risk of cancers with prasugrel than with clopidogrel was not confirmed in the TRILOGY-ACS study49 (HR=1.04; 95%CI, 0.77-1.42; P=.79; median exposure to treatment, 15 months) or in the meta-analysis by Kotronias et al.44 (HR=1.10; 95%CI, 0.89-1.37).
TicagrelorIn animals, the data are inconsistent, with experimental studies showing a potential carcinogenic role, while others show a protective function against the appearance and spread of cancer.37 The PLATO50 study found no differences in the rate of new neoplasms between ticagrelor and clopidogrel (132 vs 155; P=.17), including malignant (115 vs 121; P=.69) and benign (18 vs 35; P=.02) neoplasms. Unlike the PLATO study, the PEGASUS51 study observed a significantly higher number of cancer deaths with ticagrelor than with placebo (odds ratio=1.46; 95%CI, 1.02-2.06; P=.034), although it was not clear whether this association could be explained by the higher rate of bleeding. A recent study has found an association between ticagrelor and a lower rate of post-ACS cancer diagnosis compared with clopidogrel and prasugrel,52 although the retrospective design of this study makes it impossible to show a causal relationship between the various platelet P2Y12 receptor inhibitors and the subsequent appearance of cancer.
VorapaxarThere is no solid evidence for an association between vorapaxar and cancer in animal studies. In humans, the TRACER53 study reported 27 cancer deaths in the vorapaxar group vs 18 in the placebo group. Nevertheless, the higher number of solid cancers with vorapaxar compared with placebo that was seen in the TRACER study (HR=1.4; 95%CI, 1.1-1.8; P=.012) was not confirmed in another large study with vorapaxar, TRA2P,54 which found no differences in cancer rates between vorapaxar and placebo.
Although the long-term use of aspirin can reduce the incidence of colorectal cancer, no consistent causal association has been shown between other antiplatelet agents and a higher or lower risks of cancer. Many confounding variables affect the risk ratio between cancer and antiplatelet therapy. The higher bleeding risk associated with more aggressive antithrombotic therapy (more powerful antiplatelet drugs or longer duration of dual antiplatelet therapy) is likely to lead to diagnosis of more cancers. This could be considered positive, if an active, systematic search for cancer is undertaken in certain kinds of bleeding in patients receiving dual antiplatelet therapy, possibly allowing early diagnosis of many of these cancers and, therefore, an increased likelihood of survival in these patients.
CONCLUSIONSIn industrialized nations, cancer and CVD are the main causes of death. Up to 1 of every 10 patients with ischemic heart disease has a history of cancer, whereas 1 of every 30 patients with ischemic heart disease develops a new cancer. Both conditions share various risk factors, and the inflammatory theory is a common pathophysiologic mechanism. Although cancer can cause atherosclerosis through several mechanisms, the most common are those associated with antitumor drugs and RT, which are associated with a rather important risk of CVD and have led to a cooperative effort between cardiologists and oncologists for follow-up of oncologic patients. Additionally, there is obviously a 2-way relationship between these conditions that affects patient prognosis, as CVD is a major cause of morbidity and mortality in patients with malignant tumors and cancer doubles the risk of mortality in patients with CVD.
CONFLICTS OF INTERESTNone declared.