The MEESSI scale stratifies acute heart failure (AHF) patients at the emergency department (ED) according to the 30-day mortality risk. We validated the MEESSI risk score in a new cohort of Spanish patients to assess its accuracy in stratifying patients by risk and to compare its performance in different settings.
MethodsWe included consecutive patients diagnosed with AHF in 30 EDs during January and February 2016. The MEESSI score was calculated for each patient. The c-statistic measured the discriminatory capacity to predict 30-day mortality of the full MEESSI model and secondary models. Further comparisons were made among subgroups of patients from university and community hospitals, EDs with high-, medium- or low-activity and EDs that recruited or not patients in the original MEESSI derivation cohort.
ResultsWe analyzed 4711 patients (university/community hospitals: 3811/900; high-/medium-/low-activity EDs: 2695/1479/537; EDs participating/not participating in the previous MEESSI derivation study: 3892/819). The distribution of patients according to the MEESSI risk categories was: 1673 (35.5%) low risk, 2023 (42.9%) intermediate risk, 530 (11.3%) high risk and 485 (10.3%) very high risk, with 30-day mortality of 2.0%, 7.8%, 17.9%, and 41.4%, respectively. The c-statistic for the full model was 0.810 (95%CI, 0.790-0.830), ranging from 0.731 to 0.785 for the subsequent secondary models. The discriminatory capacity of the MEESSI risk score was similar among subgroups of hospital type, ED activity, and original recruiter EDs.
ConclusionsThe MEESSI risk score successfully stratifies AHF patients at the ED according to the 30-day mortality risk, potentially helping clinicians in the decision-making process for hospitalizing patients.
Keywords
The emergency department (ED) plays a central role in the management of acute heart failure (AHF) since about 90% of patients with this condition attend an ED to alleviate their symptoms.1 In the ED, AHF patients are usually treated with diuretic intensification, oxygen supplementation and, if needed, vasodilators and morphine.2 Once these initial treatments have been administered and their effects evaluated, a decision is needed as to whether the patient should be hospitalized or can be discharged with adequate treatment and subsequent follow-up. Although some risk scores have recently been developed for use in EDs in Canada3,4 and the United States5 with the aim of objectively supporting this decision-making process, their implementation is not widespread in Spanish EDs. Therefore, the decision to discharge patients directly from the EDs without hospitalization, currently made in about a quarter of AHF patients in Spain,2 is still empirically-driven by the subjective assessment of emergency physicians.
To address this clinical challenge, the MEESSI (Multiple Estimation of risk based on the Spanish Emergency department Score In patients with AHF) risk score was recently developed for use in AHF patients in Spanish EDs.6 This risk score demonstrates that the individual 30-day risk of mortality of AHF patients admitted to the ED can be reliably estimated using 13 readily available items. This tool has strong risk discrimination (c-statistic of 0.836), adequate model goodness-of-fit, and external validation and is now available online as a friendly website calculator.7 The MEESSI risk score also stratifies patients into 4 clinical categories, corresponding to low-, intermediate-, high- and very high-risk groups. Routine use of this risk model can potentially help clinicians to adequately manage ED admission, particularly by reliably identifying individuals at lower risk who may not require further hospitalization. However, this risk score needs to be externally validated and its accuracy checked in a new patient cohort, with particular emphasis on comparisons between the performance of MEESSI in different patient subgroups based on hospital and ED characteristics. Therefore, the aim of the present study was to validate the performance of the MEESSI risk score in a new cohort of Spanish patients and in several subgroups of hospitals and EDs, as well as to evaluate its accuracy in stratifying AHF patients by risk.
METHODSSettingWe included all consecutive patients diagnosed with AHF in the EDs of 30 Spanish hospitals (representing 9% of Spanish public hospitals) from January 1 to February 29, 2016, during Epidemiology of Acute Heart Failure in Emergency Departments (EAHFE) Registry (phase 5) patient recruitment. An extensive description of the design and recruitment dynamics of the EAHFE Registry has been extensively described elsewhere.2,6–8 In brief, patients are entered into the EAHFE Registry when AHF is diagnosed during patient stay in the ED based on the Framingham clinical diagnostic criteria.9 The principal investigator of each center makes the final adjudication based on a review of medical charts and all complementary tests done during ED stay and hospitalization. When available, each diagnosis is confirmed by natriuretic peptide determination or echocardiography following the European Society of Cardiology criteria.10 However, patients with clinical diagnostic criteria but without echocardiographic or natriuretic peptide confirmation were also included to obtain a cohort as close as possible to what is usually observed in emergency medicine practice. The only exclusion criterion was a diagnosis of ST-segment elevation myocardial infarction with concurrent development of AHF.
The cohort included in the present study is an entirely new cohort of patients not included in the previous score development and validation.6 Hospital participation in the study was by convenience and corresponded to EDs that had participated in phase 5 of the EAHFE Registry. The recruiting hospitals represent the full spectrum of Spanish health care centers attending AHF patients: university and community hospitals (n = 19/11), as well as EDs with high- (> 300 visits/d) medium- (200-300 visits/d), and low-activity (< 200 visits/d) (n = 12/12/6). Moreover, EDs previously involved in the MEESSI scale derivation study and new EDs that were not were also represented in this new cohort (n = 20/10).
The present study followed the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, and patients gave informed consent to participate and to be contacted for follow-up. The protocol was approved by the Ethics Committee at the Hospital Universitario Central de Asturias (Oviedo) Spain, with reference number 160/15.
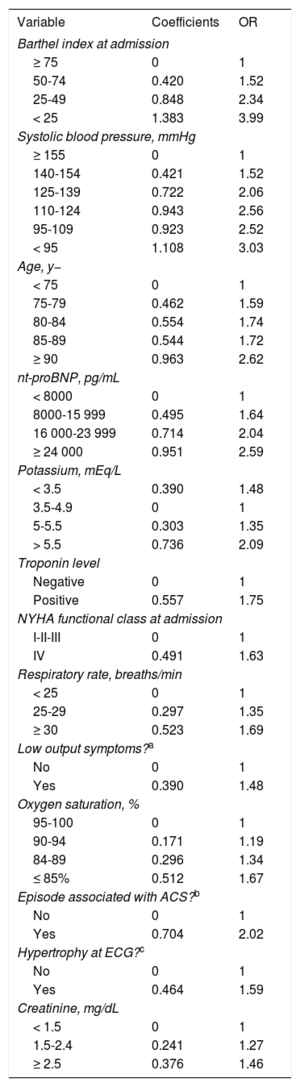
The MEESSI Risk ScoreDetails of the development and previous validation of the MEESSI risk score have been described elsewhere.6 Briefly, the score is made up of 13 risk factors (Table 1) that were originally identified from 88 candidate variables to predict 30-day all-cause mortality in patients presenting with AHF to the ED. In the final model, each continuous variable was incorporated into several ordered categories to facilitate its use in practice, and a website calculator was implemented to facilitate calculations.6,7 Furthermore, we developed 7 additional models to estimate 30-day mortality in patients lacking data of the Barthel index score, troponin level or N-terminal pro-B-type natriuretic peptide (NT-proBNP) (in any combination). The MEESSI score was retrospectively calculated for each patient included in the present study, after completion of patient management at the ED (see below).
Variables Included in the MEESSI Risk Score With the Scores for the Final Score Calculation in the Full Model and the Odds Ratio for 30-day Mortality for Each Patient Subgroup
| Variable | Coefficients | OR |
|---|---|---|
| Barthel index at admission | ||
| ≥ 75 | 0 | 1 |
| 50-74 | 0.420 | 1.52 |
| 25-49 | 0.848 | 2.34 |
| < 25 | 1.383 | 3.99 |
| Systolic blood pressure, mmHg | ||
| ≥ 155 | 0 | 1 |
| 140-154 | 0.421 | 1.52 |
| 125-139 | 0.722 | 2.06 |
| 110-124 | 0.943 | 2.56 |
| 95-109 | 0.923 | 2.52 |
| < 95 | 1.108 | 3.03 |
| Age, y− | ||
| < 75 | 0 | 1 |
| 75-79 | 0.462 | 1.59 |
| 80-84 | 0.554 | 1.74 |
| 85-89 | 0.544 | 1.72 |
| ≥ 90 | 0.963 | 2.62 |
| nt-proBNP, pg/mL | ||
| < 8000 | 0 | 1 |
| 8000-15 999 | 0.495 | 1.64 |
| 16 000-23 999 | 0.714 | 2.04 |
| ≥ 24 000 | 0.951 | 2.59 |
| Potassium, mEq/L | ||
| < 3.5 | 0.390 | 1.48 |
| 3.5-4.9 | 0 | 1 |
| 5-5.5 | 0.303 | 1.35 |
| > 5.5 | 0.736 | 2.09 |
| Troponin level | ||
| Negative | 0 | 1 |
| Positive | 0.557 | 1.75 |
| NYHA functional class at admission | ||
| I-II-III | 0 | 1 |
| IV | 0.491 | 1.63 |
| Respiratory rate, breaths/min | ||
| < 25 | 0 | 1 |
| 25-29 | 0.297 | 1.35 |
| ≥ 30 | 0.523 | 1.69 |
| Low output symptoms?a | ||
| No | 0 | 1 |
| Yes | 0.390 | 1.48 |
| Oxygen saturation, % | ||
| 95-100 | 0 | 1 |
| 90-94 | 0.171 | 1.19 |
| 84-89 | 0.296 | 1.34 |
| ≤ 85% | 0.512 | 1.67 |
| Episode associated with ACS?b | ||
| No | 0 | 1 |
| Yes | 0.704 | 2.02 |
| Hypertrophy at ECG?c | ||
| No | 0 | 1 |
| Yes | 0.464 | 1.59 |
| Creatinine, mg/dL | ||
| < 1.5 | 0 | 1 |
| 1.5-2.4 | 0.241 | 1.27 |
| ≥ 2.5 | 0.376 | 1.46 |
ACS, acute coronary syndrome; ECG, electrocardiogram; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; OR, odds ratio.
To calculate the MEESSI score for a particular patient, the 13 coefficients must be added together plus the intercept coefficient, which is −5.399.
We recorded 56 variables (including the 13 needed to calculate the MEESSI score): 2 on epidemiology, 12 on comorbidity, 10 on chronic treatments received at home, 1 on echocardiographic data, 6 on precipitants of the current episode of AHF, 6 on clinical status at ED arrival, 4 on electrocardiogram results, 7 on laboratory data, and 8 on treatment and management at the ED. The data collection process and protocols were the same as those used in the previous EAHFE registries.2,6–8 Since this was an observational study, no intervention was tested; hence our data reflect the usual patient management provided by the attending physicians. During the time of patient recruitment, the MEESSI risk score was not yet available, and all decisions on patient management were carried out without knowledge of the patient risk category. In addition to 30-day mortality, which is the outcome estimated by the MEESSI scale, 30-day ED revisits and 30-day hospitalization due to AHF were also recorded.
Statistical AnalysisQualitative variables are expressed as frequency and percentage, and continuous variables as mean ± standard deviation or, if not normally distributed, as median and [interquartile range]. Comparisons were carried out using chi-square, ANOVA or Kruskal-Wallis tests, respectively. A multiple imputation technique using chained equations11 was used to produce 50 imputed data sets replacing the missing values in the 13 variables included in the MEESSI risk score. Using the imputed data, we calculated the individual MEESSI risk score (x) for each patient, adding up their relevant coefficient for each risk factor on top of the intercept value (−5.40). To estimate the probability of death within 30 days, we applied the inverse of the logit function [ex/(1+ex)]. Patients were stratified into 4 clinical risk categories (low/intermediate/high/very high), which were produced using thresholds from the original MEESSI risk score stratification and correspond to the 2 bottom quintiles (low risk), the third and fourth quintiles (intermediate risk), and the ninth and tenth deciles (high risk and very high risk) of mortality. The time-to-first-event curve for 30-day mortality was obtained for each risk category using the Kaplan-Meier method and compared using the log-rank test. In this validation cohort, a goodness-of-fit model was assessed plotting the observed vs the predicted risk of mortality as well as the Hosmer-Lemeshow test. The rates of 30-day ED revisit and hospitalization due to AHF was calculated for each risk category. A receiver operating characteristic curve (c-statistic) using the MEESSI risk score was performed as a measure of classifier performance for the complete model and for the 7 models derived from the lack of Barthel index score, troponin level or NT-proBNP (in any combination). As a sensitivity analysis, we repeated this analysis using observed data (without imputing missing data, complete-case analysis) and assessed the c-statistic for the 8 models in this reduced sample. Finally, stratified analyses using the full model were performed by applying the risk score to patients recruited in university and community hospitals, to patients recruited in high-, medium- and low-activity EDs, and to patients included by EDs participating and not participating in patient recruitment for the original derivation of the MEESSI risk score. STATA software, version 13.1 (Stata Corp, College Station, Texas, United States) was used for the analyses.
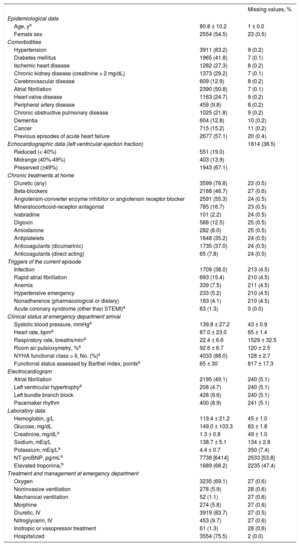
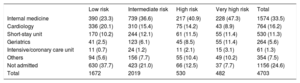
RESULTSWe studied 4711 new patients (2 were excluded from the original 4713 patients due to lack of follow-up) recruited in 19 university and 11 community hospitals (3811 and 900 cases, respectively) in EDs with high-, medium- and low-activity (2695, 1479, and 537 cases, respectively) and in EDs that did or did not participate in the original derivation of the MEESSI (3892 and 819 cases, respectively). Of note, patients were of advanced age (mean 80.8 years ± 10.2), with multiple comorbidities (the most frequent being hypertension in 83.2%, atrial fibrillation in 50.8%, and diabetes mellitus in 41.8%), more than half had had previous episodes of AHF, and two-thirds had preserved left ventricular ejection fraction (> 49%). The remaining clinical characteristics are shown in Table 2. There was less than 10% of missing values in 50 out of the 56 clinical variables (89.3%), and only echocardiographic data, respiratory rate, Barthel index at arrival, potassium, NT-proBNP and troponin levels showed higher proportions of missingness. The final patient destination after ED care is shown in Table 3, with the main destinations for admitted patients (75.4% of cases) being internal medicine and cardiology wards, although the distribution significantly differed according to the risk category (P < .001).
Clinical Characteristics of the 4711 New Patients With Acute Heart Failure Included in the Present Study
| Missing values, % | ||
|---|---|---|
| Epidemiological data | ||
| Age, ya | 80.8 ± 10.2 | 1 ± 0.0 |
| Female sex | 2554 (54.5) | 23 (0.5) |
| Comorbidities | ||
| Hypertension | 3911 (83.2) | 9 (0.2) |
| Diabetes mellitus | 1965 (41.8) | 7 (0.1) |
| Ischemic heart disease | 1282 (27.3) | 8 (0.2) |
| Chronic kidney disease (creatinine > 2 mg/dL) | 1373 (29.2) | 7 (0.1) |
| Cerebrovascular disease | 609 (12.9) | 8 (0.2) |
| Atrial fibrillation | 2390 (50.8) | 7 (0.1) |
| Heart valve disease | 1163 (24.7) | 9 (0.2) |
| Peripheral artery disease | 459 (9.8) | 8 (0.2) |
| Chronic obstructive pulmonary disease | 1025 (21.8) | 9 (0.2) |
| Dementia | 604 (12.8) | 10 (0.2) |
| Cancer | 715 (15.2) | 11 (0.2) |
| Previous episodes of acute heart failure | 2677 (57.1) | 20 (0.4) |
| Echocardiographic data (left ventricular ejection fraction) | 1814 (38.5) | |
| Reduced (< 40%) | 551 (19.0) | |
| Midrange (40%-49%) | 403 (13.9) | |
| Preserved (≥49%) | 1943 (67.1) | |
| Chronic treatments at home | ||
| Diuretic (any) | 3599 (76.8) | 23 (0.5) |
| Beta-blockers | 2188 (46.7) | 27 (0.6) |
| Angiotensin-converter enzyme inhibitor or angiotensin receptor blocker | 2591 (55.3) | 24 (0.5) |
| Mineralocorticoid-receptor antagonist | 785 (16.7) | 23 (0.5) |
| Ivabradine | 101 (2.2) | 24 (0.5) |
| Digoxin | 588 (12.5) | 25 (0.5) |
| Amiodarone | 282 (6.0) | 25 (0.5) |
| Antiplatelets | 1648 (35.2) | 24 (0.5) |
| Anticoagulants (dicumarinic) | 1735 (37.0) | 24 (0.5) |
| Anticoagulants (direct acting) | 65 (7.8) | 24 (0.5) |
| Triggers of the current episode | ||
| Infection | 1709 (38.0) | 213 (4.5) |
| Rapid atrial fibrillation | 693 (15.4) | 210 (4.5) |
| Anemia | 339 (7.5) | 211 (4.5) |
| Hypertensive emergency | 233 (5.2) | 210 (4.5) |
| Nonadherence (pharmacological or dietary) | 183 (4.1) | 210 (4.5) |
| Acute coronary syndrome (other than STEMI)a | 63 (1.3) | 0 (0.0) |
| Clinical status at emergency department arrival | ||
| Systolic blood pressure, mmHga | 139.8 ± 27.2 | 43 ± 0.9 |
| Heart rate, bpma | 87.0 ± 23.0 | 65 ± 1.4 |
| Respiratory rate, breaths/mina | 22.4 ± 6.6 | 1529 ± 32.5 |
| Room air pulsioxymetry, %a | 92.6 ± 6.7 | 120 ± 2.5 |
| NYHA functional class > II, No. (%)a | 4033 (88.0) | 128 ± 2.7 |
| Functional status assessed by Barthel index, pointsa | 65 ± 30 | 817 ± 17.3 |
| Electrocardiogram | ||
| Atrial fibrillation | 2195 (49.1) | 240 (5.1) |
| Left ventricular hypertrophya | 208 (4.7) | 240 (5.1) |
| Left bundle branch block | 428 (9.6) | 240 (5.1) |
| Pacemaker rhythm | 400 (8.9) | 241 (5.1) |
| Laboratory data | ||
| Hemoglobin, g/L | 119.4 ± 21.2 | 45 ± 1.0 |
| Glucose, mg/dL | 149.0 ± 103.3 | 83 ± 1.8 |
| Creatinine, mg/dLa | 1.3 ± 0.8 | 49 ± 1.0 |
| Sodium, mEq/L | 138.7 ± 5.1 | 134 ± 2.8 |
| Potassium, mEq/La | 4.4 ± 0.7 | 350 (7.4) |
| NT-proBNP, pg/mLa | 7738 [6414] | 2533 [53.8] |
| Elevated troponina,b | 1689 (68.2) | 2235 (47.4) |
| Treatment and management at emergency department | ||
| Oxygen | 3235 (69.1) | 27 (0.6) |
| Noninvasive ventilation | 278 (5.9) | 28 (0.6) |
| Mechanical ventilation | 52 (1.1) | 27 (0.6) |
| Morphine | 274 (5.8) | 27 (0.6) |
| Diuretic, IV | 3919 (83.7) | 27 (0.5) |
| Nitroglycerin, IV | 453 (9.7) | 27 (0.6) |
| Inotropic or vasopressor treatment | 61 (1.3) | 28 (0.6) |
| Hospitalized | 3554 (75.5) | 2 (0.0) |
IV, intravenous; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; STEMI, ST-segment elevation myocardial infarction.
Values are expressed as No. (%), mean ± standard deviation, or median [interquartile range].
Final Destination of Patients Diagnosed With Acute Heart Failure in the Emergency Department According to the Risk Category Assigned by the MEESSI Scale
| Low risk | Intermediate risk | High risk | Very high risk | Total | |
|---|---|---|---|---|---|
| Internal medicine | 390 (23.3) | 739 (36.6) | 217 (40.9) | 228 (47.3) | 1574 (33.5) |
| Cardiology | 336 (20.1) | 310 (15.4) | 75 (14.2) | 43 (8.9) | 764 (16.2) |
| Short-stay unit | 170 (10.2) | 244 (12.1) | 61 (11.5) | 55 (11.4) | 530 (11.3) |
| Geriatrics | 41 (2.5) | 123 (6.1) | 45 (8.5) | 55 (11.4) | 264 (5.6) |
| Intensive/coronary care unit | 11 (0.7) | 24 (1.2) | 11 (2.1) | 15 (3.1) | 61 (1.3) |
| Others | 94 (5.6) | 156 (7.7) | 55 (10.4) | 49 (10.2) | 354 (7.5) |
| Not admitted | 630 (37.7) | 423 (21.0) | 66 (12.5) | 37 (7.7) | 1156 (24.6) |
| Total | 1672 | 2019 | 530 | 482 | 4703 |
Eight missing values (with no knowledge as to where the patients were admitted, 0.17%).
Values are expressed as No. (%).
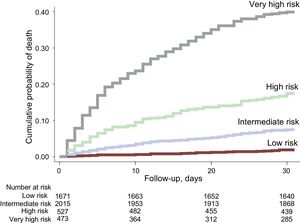
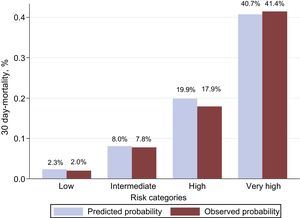
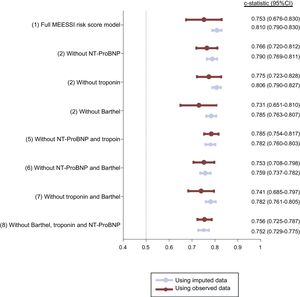
In this validation cohort, 486 (10.3%) patients died within 30 days of ED admission. The MEESSI risk score predicted 30-day mortality using 13 variables (full model) with excellent discrimination (c-statistic, 0.810; 95% confidence interval, 0.790-0.830; P < .001).The distribution of patients across the clinical risk categories defined by the MEESSI score was as follows: 1673 (35.5%) were classified as low risk, 2023 (42.9%) as intermediate risk, 530 (11.3%) as high risk, and 485 (10.3%) as very high risk. The steep-gradient in 30-day mortality remained consistent across risk categories, with cumulative mortalities at 30 days of 2.0%, 7.8%, 17.9%, and 41.4%, respectively (Figure 1). The goodness-of-fit of the model was excellent (Figure 2). The other 7 models for the MEESSI risk score, which can be applied to predict 30-day mortality in the absence of the Barthel index, NT-proBNP and/or troponin, showed c-statistics between 0.752 and 0.806. Our sensitivity analysis using only patients with complete data (ie, not using imputed values for those variables lacking data) rendered similar values for the 8 models, with c-statistics ranging from 0.731 to 0.785 (Figure 3). In addition, the 30-day revisit to the ED was higher with an increase in the risk category (18.5%, 23.5%, 23.7%, and 27.0%, respectively, P = .01), and the same relationship was found with respect to 30-day rehospitalization (11.0%, 17.5%, 19.7%, and 22.5%; P < .001).
Kaplan-Meier curves showing the 30-day cumulative mortality for the 4 clinical risk groups defined by the MEESSI risk score. Low-, intermediate-, high- and very high-risk categories correspond to groups defined by the cutoffs found in the original derivation study that included the first and second bottom quintiles, the third and fourth quintiles, the ninth decile, and the top decile of 30-day mortality, respectively.
Assessment of the goodness-of-fit of the MEESSI scale in the new patients included in the present study by comparing observed and predicted 30-day mortality. The Hosmer-Lemeshow test (P = .745) indicates that the 30-day mortality estimated by the MEESSI risk score does not significantly deviate from the observed data.
Description of the area-under-the-curve receiver operating characteristic for the full MEESSI-AHF model and for each reduced model using imputed and real data. Estimates in the group “Using imputed data” were performed with all the 4711 patients in all the models, and included real values and also imputed values for missing data. Estimates in the group “Using observed data” were performed using only patients with complete data for all relevant variables: 602 patients in model 1 (full MEESSI risk score model); 1215 patients in model 2; 953 patients in model 3; 662 patients in model 4; 2216 patients in model 5; 1394 patients in model 6; 1048 patients in model 7, and 2669 patients in model 8. 95%CI, 95% confidence interval; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
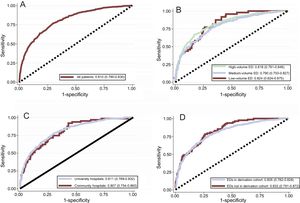
Figure 4 shows the subgroup analysis of the discriminatory capacity of the MEESSI risk score by type of hospital, ED activity volume, and previous participation in score derivation. Very similar c-statistics were obtained with no significant differences among subgroups. Remarkably, the discriminatory capacity of our model was very high (c-statistic: 0.832; 95% confidence interval, 0.791-0.872) in the sample of patients recruited in EDs which did not participate in the derivation of the MEESSI risk score.
Receiver operating characteristic curves for the MEESSI risk score applied to the complete cohort (A), and for the subgroups of patients from university and community hospitals (B), high-, medium- and low-activity EDs (C), and from EDs that did and did not participate in the original derivation of the MEESSI risk score (D). Receiver operating characteristic curves were obtained with the full MEESSI model and included all patients (those with complete real data and those with some imputation because of missing data). ED, emergency department.
We have externally validated the MEESSI risk score in a new cohort of AHF patients from 30 Spanish EDs. The results demonstrate that the MEESSI score has a high discriminatory capacity, which is consistent across several subgroup analyses, including 10 EDs that were not previously involved in the derivation process. The MEESSI scale classifies patients into 4 clinical risk categories. In particular, 35.7% patients were allocated to the low-risk category (cumulative 30-day mortality of 2.0%), and 10.1% were classified into the very high-risk category (30-day cumulative mortality of 41.4%, more than 20-fold higher than low-risk patients). This clear-cut risk stratification based on the prediction of 30-day mortality may help emergency physicians make clinical decisions with respect to AHF patient disposition based on objective risk estimates.
The MEESSI risk score showed a remarkably high discriminatory capacity across 3 cohorts of Spanish patients (c-statistic of 0.836 and 0.828 in the derivation and validation original cohorts, and 0.810 in the current validation study). To our knowledge, only 3 risk scales derived from ED patients have previously been developed to predict mortality: 1 was from the United States (The STRATIFY [Improving Heart Failure Risk Stratification in the Emergency Department]5) and 2 were from Canada (the Ottawa Heart Failure Risk Scale [OHFRS],3 and the Emergency Heart Failure Mortality Risk Grade, [EHMRG]4). However, their discriminatory capacity was lower than that observed with the MEESSI risk score (c-statistics 0.68 for STRATIFY, 0.77 for OHFRS, and 0.807/0.804 for the EHMRG derivation and validation cohorts, respectively). Moreover, since risk scores work with the populations to which they are applied, differences in health care system characteristics and local organization could have an impact on their performance. This is particularly true for AHF in which transition plans, outpatient clinics, walk-in centers, AHF clinics and other specifically devoted disease pathways play an important role in patient management and may influence outcomes.12–14 On testing the performance of the EHMRG score in 2 different Spanish cohorts of 1553 and 2137 AHF ED patients, the c-statistic decreased from the original values (0.807/0.804) to 0.74115 and 0.750,6 respectively. Taking into account that we included all consecutive AHF patients and only excluded ST-segment elevation myocardial infarction patients with concomitant AHF, the MEESSI risk score can potentially be applied to the vast majority of AHF patients diagnosed in EDs. This also makes our risk score different from the remaining scores available, since some were developed excluding substantial subsets of patients (ie, EHMRG excluded palliative patients and OHFRS enrolled a nonconsecutive sample with multiple exclusion criteria). Therefore, our results suggest that the MEESSI risk score not only performs well in Spanish EDs but also covers a global unmet clinical need.
The marked accuracy of the MEESSI risk score for identifying low-risk patients is potentially clinically relevant. We found that patients with AHF discharged to home from Spanish EDs without hospitalization were exposed to a significantly increased risk of adverse outcomes, which is especially high in the short-term. Indeed, the hazard ratios for ED- compared with hospital-discharged patients, after adjustment for patient and center features, was 2.07 (95% confidence interval, 1.19-3.60) for the 7-day ED revisit, and 3.07 (95% confidence interval, 1.92-4.92) for the 7-day hospitalization risk.16 Similar results have been reported in Canada17,18 and the United States.19 This clearly underlines that there is room for improvement in ED decision-making and that the definition and implementation of effective strategies to improve patient selection for direct ED discharge can improve outcomes. In a recent consensus document, experts proposed that in EDs in which extended observation is possible (usually up to 24hours), such as in many Spanish EDs, the direct ED discharge rate should be higher than 40% while maintaining a 7-day revisit rate of less than 10%, a 30-day mortality of less than 2%, and a combined 30-day ED revisit or rehospitalization rate of less than 20% for this subgroup of patients managed without hospitalization.20 The MEESSI score stratified nearly 40% of all patients in the low-risk group, and the 30-day mortality was approximately 2% in this group. In addition, our data suggest that the MEESSI scale could also stratify the risk of a revisit to ED or rehospitalization due to AHF, although this potential capacity will have to be tested in further studies. These figures suggest that it should be feasible to achieve the goals of the previously mentioned expert recommendations, and the MEESSI score could help to achieve these objectives. However, as a large proportion (62.3%) of low-risk patients were hospitalized, it is not known if the good outcomes achieved in these low-risk patients would have remained the same if these patients had been discharged directly from the ED to home without hospitalization.
The detection of AHF patients at very high risk can also assist emergency physicians to better recognize this subset of patients, who have a strong probability of dying within the days following ED admission. The lack of immediate identification of these patients and the delay in offering them intensive care is, in fact, associated with poorer outcomes.21,22 In this regard, the MEESSI risk score could also help to improve outcomes in this challenging patient subgroup, although other risk scales developed in the ED arena can be applied in this setting, such as the recently published EAHFE-3D,23,24 which could even be more successful in these very sick patients. Nonetheless, the early detection of this situation does not necessarily lead to patient admission in intensive care units, since palliative rather than intensive care may be the most adequate approach in some patients in whom AHF is the final stage of chronic advanced cardiomyopathy.25,26 In fact, our data show that most of the patients allocated to the very high-risk category were not admitted to cardiology and intensive/coronary care units, suggesting that a high proportion of these patients were allocated to palliative care.
LimitationsOur study has some limitations. First, as for any single-country study, caution should be exercised in extrapolating the findings internationally. Moreover, the EDs were not randomly selected but were participants of the EAHFE Registry, including local investigators with a special interest in AHF. However, the performance of MEESSI in patients from EDs newly participating in the EAHFE Registry that had not been previously involved in the development of the MEESSI score was very good. We believe that similar results could be obtained if applied to other EDs,27 at least in Spain. Second, some variables, such as the Barthel index, New York Heart Association functional class, association with acute coronary syndrome, or low cardiac output, are partially based on subjective interpretation, and adjudication can vary between different observers. Third, the MEESSI score only evaluates the 30-day risk of mortality. While it could be argued that other relevant outcomes, such as ED revisit or the need for rehospitalization could make this estimate more realistic or meaningful, the relevance of including some of these outcomes to estimate AHF risk is still a matter of debate. Moreover, the causes of death were not differentiated; therefore, the real contribution of cardiovascular causes to fatal events is unknown. Fourth, being a real-world observational study, some important predictors had a high number of missing values. Nonetheless, this issue has been addressed using the multiple imputation technique and presenting 7 additional models that allow risk estimation in the absence of information on the Barthel index, NT-proBNP, or troponin. However, these measures can easily be prospectively obtained in most cases, with the exception perhaps of NT-proBNP, and therefore, it should be feasible to calculate the full MEESSI model most of the time. Fifth, variables related to prehospital management by emergency medical teams were not taken into account, and there is increasing evidence that prehospital care can impact outcomes.28,29 Finally, the accuracy of MEESSI risk estimation could change in the future if new prognosis-modifying treatments for heart failure become available. For example, the currently available angiotensin receptor blocker neprilysin inhibitors were not included in the development and validation of the MEESSI scale.
CONCLUSIONSThe MEESSI risk score is a clinically useful tool for acute risk stratification of AHF patients in all Spanish EDs and eventually worldwide. It has been carefully developed in a large, multicenter cohort, including virtually all kinds of AHF patients. It has been internally and externally validated, showing good performance using only variables readily available in the ED arena. In addition, online access to a user-friendly risk calculator that works even in the absence of some variables6 makes it very practical. We hope that the generalization of accurate risk estimation of patients with AHF during ED care by emergency physicians will help to improve early clinical decision-making and, in turn, positively impact short-term outcomes in a syndrome whose prognosis has remained largely unchanged over several decades.
FUNDINGThis study was partially supported by grants from the Instituto de Salud Carlos III supported with funds from the Spanish Ministry of Health and FEDER (PI15/01019 and PI15/00773) and Fundació La Marató de TV3 (2015/2510). The “Emergencies: Processes and Pathologies” research group of the IDIBAPS receives financial support from the Catalonian Government for Consolidated Groups of Investigation (GRC 2009/1385 and 2014/0313). Xavier Rosselló has received support from the SEC-CNIC CARDIOJOVEN fellowship program.
CONFLICTS OF INTERESTThe ICA-SEMES Research Group has received unrestricted support from Orion Pharma and Novartis. The present study was designed, performed, analyzed, and written exclusively by the authors independently of these pharmaceutical companies. H. Bueno reports grants and personal fees from Astrazeneca, Daiichi Sankyo, Eli Lilly, Bayer, Sanofi during the conduct of the study; personal fees from Novartis, BMS-Pfizer, Servier outside the submitted work.
- –
Decision-making (discharge or hospitalization) of patients with AHF attended at the ED is still made without risk stratification.
- –
Only a few scales have been developed based on patients diagnosed with AHF, all being from the United States and Canada, and none is currently in use.
- –
The results of the present study validate the performance of the MEESSI risk score in a new cohort of Spanish patients and in several subgroups of hospitals and EDs and also evaluated the accuracy of this score in stratifying AHF patients according to risk.
- –
The MEESSI risk score shows that physicians can use 13 readily available items to estimate the individual risk of death within 30 days for patients with AHF who are admitted to the ED.
- –
This tool has excellent discrimination and calibration and was validated in a cohort different from that used to develop the score.
- –
Physicians can consider using this tool to make clinical decisions.
- –
The MEESSI risk score is a clinically useful tool for acute risk stratification of AHF patients in all Spanish EDs, and eventually, worldwide.
We thank Alícia Díaz for her professionalism in data management. Other investigators of the ICA-SEMES (Research group on Acute Heart Failure of the Spanish Society of Emergency Medicine) can be consulted at the