The predictive value of the SYNTAX score (SS) for clinical outcomes after transcatheter aortic valve implantation (TAVI) is very limited and could potentially be improved by the combination of anatomic and clinical variables, the SS-II. We aimed to evaluate the value of the SS-II in predicting outcomes in patients undergoing TAVI.
MethodsA total of 402 patients with severe symptomatic aortic stenosis undergoing transfemoral TAVI were included. Preprocedural TAVI angiograms were reviewed and the SS-I and SS-II were calculated using the SS algorithms. Patients were stratified in 3 groups according to SS-II tertiles. The coprimary endpoints were all-cause death and major adverse cardiovascular events (MACE), a composite of all-cause death, cerebrovascular event, or myocardial infarction at 1 year.
ResultsIncreased SS-II was associated with higher 30-day mortality (P=.036) and major bleeding (P=.015). The 1-year risk of death and MACE was higher among patients in the 3rd SS-II tertile (HR, 2.60; P=.002 and HR, 2.66; P<.001) and was similar among patients in the 2nd tertile (HR, 1.27; P=.507 and HR, 1.05; P=.895) compared with patients in the 1st tertile. The highest SS-II tertile was an independent predictor of long-term mortality (P=.046) and MACE (P=.001).
ConclusionsThe SS-II seems more suited to predict clinical outcomes in patients undergoing TAVI than the SS-I. Increased SS-II was associated with poorer clinical outcomes at 1 and 4 years post-TAVI, independently of the presence of coronary artery disease.
Keywords
Aortic stenosis frequently coexists with coronary artery disease (CAD), sharing common risk factors and a similar pathogenesis.1,2 The risk of surgical aortic valve replacement is increased in the presence of CAD, with coronary artery bypass graft (CABG) generally indicated at the time of valve surgery.3 Transcatheter aortic valve implantation (TAVI) has revolutionized the treatment of intermediate and high-risk patients with severe symptomatic aortic stenosis.4 The extent and complexity of CAD in these patients is heterogeneous and optimal management remains unknown. Furthermore, with the expansion of TAVI in clinical practice, the ability to predict postprocedural outcomes, particularly in lower-risk patients, is of increasing interest. Previous studies have reported that the presence of CAD in TAVI candidates had no impact on short- and mid-term clinical outcomes. The anatomic SYNTAX score I (SS-I), originally designed to assess the procedural complexity of percutaneous coronary revascularization,5 has been investigated as a potential tool to predict major cardiovascular events in patients undergoing TAVI.6,7 However, the predictive value of the SS-I was found to be very limited, potentially as a result of focusing only on coronary anatomy, ignoring the important role of patient comorbidities. A more recent index, the SYNTAX score II (SS-II), which combines anatomic characteristics with clinical variables, has been shown to provide a long-term, individualized risk assessment for patients with complex CAD.8–10 Although the SS-II was developed to aid in decision-making between CABG and percutaneous coronary intervention (PCI) in patients with complex CAD, we hypothesized that the inclusion of clinical variables, several of which have been identified as predictors of increased mortality in surgical aortic valve replacement11,12 and TAVI13,14 may improve the prognostic value over that of the SS-I in predicting outcomes post-TAVI.
METHODSPatient PopulationA total of 402 consecutive patients with severe symptomatic aortic stenosis undergoing transfemoral TAVI at our institution were included. The indication for TAVI was reviewed in the context of the institutional Heart Team meeting, including at least 1 cardiac surgeon and interventional, imaging and clinical cardiologists, based on the patient's clinical history, anatomical suitability and frailty assessment. Severe aortic stenosis was defined as a mean transaortic pressure gradient of >40mmHg or valve area of<1.0cm2 using transthoracic, transoesophageal or dobutamine stress echocardiography, as appropriate. All patients underwent routine coronary angiography prior to TAVI. The decision to perform coronary revascularization was made at the Heart Team meeting, based on the location and extent of the CAD and complexity of PCI. In general, proximal and mid severe lesions with a large amount of myocardium at risk were revascularized and lesions in distal segments or secondary vessels were not treated. Patients underwent replacement of the Edwards Sapien transcatheter heart valve (Edwards LifeSciences, Irvine, California, United States) or the Medtronic CoreValve bioprosthesis (Medtronic, Minneapolis, Minnesota, United States) using the transfemoral route, as previously described.15 Electrocardiogram and cardiac markers were monitored every 8hours during the first 24hours and daily afterwards, in all patients as part of our standard TAVI protocol. Antithombotic treatment after TAVI consisted of aspirin (indefinitely) plus clopidogrel (3-6 months) unless contraindicated. If anticoagulation was indicated for any other reason, oral anticoagulant therapy was administrated (with or without single antiplatelet therapy). All patients provided informed consent for the procedure and follow-up.
Angiographic AnalysisPreprocedural angiograms were analyzed in the angiographic core laboratory of our institution by an interventional cardiologist expert in the assessment of the SS-I and blinded to clinical outcomes.16 CAD was defined as the presence of 1 or more lesions of the epicardial coronary arteries with ≥ 50% diameter stenosis in vessels ≥ 1.5mm in diameter.17 The SS-I was calculated using the SS-I algorithm.17 In patients with previously revascularized lesions, which remained patent at the time of the pre-TAVI coronary angiogram, these lesions were considered to be nonsignificant in the calculation of the SS-I, but these patients were included in the CAD group. When previous revascularization was carried out surgically, the SS-I score was calculated using the CABG SYNTAX score.18 A residual SYNTAX score was calculated in patients who underwent PCI prior to TAVI.19 The SS-II was calculated for all patients using the SS-II algorithm,8 which includes the anatomical SS-I and baseline clinical variables such as age, sex, creatinine clearance, left ventricle ejection fraction, left main disease, chronic obstructive pulmonary disease, and peripheral vascular disease. In patients who underwent PCI in the 30 days prior to TAVI, the pre-PCI SS-I was used and, in patients without coronary lesions, an SS-I value of 0 was entered.
Study Endpoints and DefinitionsThe coprimary endpoints of this study were all-cause death and major adverse cardiovascular events, a composite of all-cause death, nonfatal cerebrovascular event, or nonfatal myocardial infarction at 1 year. Secondary endpoints were the individual components of the primary endpoint at 1 year, as well as cardiovascular death. Major adverse cardiovascular events and their components were assessed at 30 days, 1 year, and 4 years. Cardiovascular death was defined as any death due to a cardiac cause or death of unknown cause, all procedure-related deaths (defined as all-cause mortality within 30 days or during the index procedure hospitalization if longer than 30 days), and deaths due to cerebrovascular disease, pulmonary embolism, or vascular disease.20 Cerebrovascular events included transient ischemic attack and stroke defined according to symptom duration, persistent neurological dysfunction, and/or evidence of cerebral infarction on imaging.20 Myocardial injury was defined as evidence of new Q waves on the electrocardiogram or new regional wall motion abnormalities at echocardiography. Biochemical markers of myocardial injury were defined as a rise in troponin >35 upper limit normal (ULN) or creatinine kinase-isoenzyme MB >5 ULN.
Clinical Follow-upAdverse events were assessed in hospital and at clinical follow-up. Baseline clinical and procedural characteristics and adverse events were prospectively entered into the dedicated institutional database. Patients were followed up at 1 month, 6 months, and 1 year postprocedure and yearly thereafter.
Statistical AnalysisBecause the SS-II produces estimates of mortality for both PCI and CABG, a C statistic for 30-day mortality and 1-year mortality was carried out using binary logistic regression analyses. Receiver operating characteristic (ROC) curves were constructed and pairwise comparison of ROC curves among SS-I, SS-II PCI, and SS-II CABG was performed to assess the best score for the identification of the primary endpoint. In addition, time-dependent ROC curves for censored survival data were also created to assess the predictive value of both SS-II PCI and CABG for 1-year mortality.21 The SS-II for PCI proved the best fit and therefore all further analysis was carried out using this score. Patients were stratified into 3 groups according to SS-II PCI tertiles. Baseline characteristics and clinical outcomes were compared between groups. Categorical variables are summarized as counts and frequencies and continuous data are presented as means ± standard deviation or median [interquartile range (IQR), 25th-75th percentile] when not normally distributed, using the Shapiro-Wilk test. Clinical outcomes at 30 days, 1 year, and 4 years are expressed as counts or incidence rates using Kaplan-Meier analysis and compared between patients in the 1st SS-II tertile, 2nd SS-II tertile, and 3rd SS-II tertile. Analyses were also tested in patients with and without CAD according to SS-II PCI tertiles. P values<.05 were considered statistically significant. The analyses were performed using SPSS 19.0 for Windows (SPSS, Inc, Chicago, Illiniois, United States) and R statistical software, version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
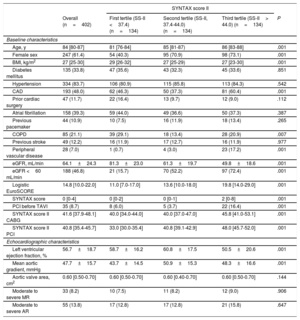
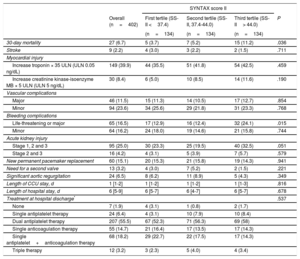
RESULTSBaseline and Procedural CharacteristicsBaseline clinical characteristics are summarized in Table 1. Overall, the median age was 84 [IQR 80-87] years with a high prevalence of diabetes (34%), atrial fibrillation (39%), and renal failure (47%). A total of 193 (48.0%) patients had CAD with a median SS-I and residual SS-I of 4 [IQR, 0-9] and 2 [IQR, 0-7], respectively. The areas under the ROC curves for SS-I and residual SS-I were 0.520 (95%CI, 0.404–0.636; P=.732) and 0.510 (95%CI, 0.398–0.623; P=.856) for 30-day mortality and 0.551 (95%CI, 0.473–0.629; P=.185) and 0.524 (95%CI, 0.448–0.601; P=.527) for 1-year mortality, respectively. The overall median SS-II for PCI and CABG was 40.8 [IQR, 35.4-45.7] and 41.6 [IQR, 37.9-48.1], respectively. The SS-II for PCI proved a better fit than the SS-II for CABG for both 30-day (C statistic, 0.632; 95%CI, 0.524-0.740; P=.022; C statistic, 0.480; 95%CI, 0.380-0.580; P=.728, respectively, P=.032 for comparison of both ROC curves) and 1-year mortality (C statistic 0.625; 95%CI, 0.552-0.699; P=.001; C statistic, 0.539; 95%CI, 0.466-0.612; P=.309, respectively, P=.075 for comparison of both ROC curves) (). The time-dependent area under curve (AUC) also showed better fit with SS-II for PCI compared with the SS-II for CABG within 1 year (). Therefore all further analysis was carried out using the SS-II for PCI according to its tertiles: 1st SS-II tertile (< 37.4), 2nd SS-II tertile (37.4-44.0), and 3rd SS-II tertile (> 44).
Baseline Clinical and Echocardiographic Characteristics According to SYNTAX Score II Tertiles
| SYNTAX score II | |||||
|---|---|---|---|---|---|
| Overall (n=402) | First tertile (SS-II <37.4) (n=134) | Second tertile (SS-II, 37.4-44.0) (n=134) | Third tertile (SS-II> 44.0) (n=134) | P | |
| Baseline characteristics | |||||
| Age, y | 84 [80-87] | 81 [76-84] | 85 [81-87) | 86 [83-88] | .001 |
| Female sex | 247 (61.4) | 54 (40.3) | 95 (70.9) | 98 (73.1) | .001 |
| BMI, kg/m2 | 27 [25-30] | 29 [26-32] | 27 [25-29) | 27 [23-30] | .001 |
| Diabetes mellitus | 135 (33.8) | 47 (35.6) | 43 (32.3) | 45 (33.6) | .851 |
| Hypertension | 334 (83.7) | 106 (80.9) | 115 (85.8) | 113 (84.3) | .542 |
| CAD | 193 (48.0) | 62 (46.3) | 50 (37.3) | 81 (60.4) | .001 |
| Prior cardiac surgery | 47 (11.7) | 22 (16.4) | 13 (9.7) | 12 (9.0) | .112 |
| Atrial fibrillation | 158 (39.3) | 59 (44.0) | 49 (36.6) | 50 (37.3) | .387 |
| Previous pacemaker | 44 (10.9) | 10 (7.5) | 16 (11.9) | 18 (13.4) | .265 |
| COPD | 85 (21.1) | 39 (29.1) | 18 (13.4) | 28 (20.9) | .007 |
| Previous stroke | 49 (12.2) | 16 (11.9) | 17 (12.7) | 16 (11.9) | .977 |
| Peripheral vascular disease | 28 (7.0) | 1 (0.7) | 4 (3.0) | 23 (17.2) | .001 |
| eGFR, mL/min | 64.1±24.3 | 81.3±23.0 | 61.3±19.7 | 49.8±18.6 | .001 |
| eGFR <60 mL/min | 188 (46.8) | 21 (15.7) | 70 (52.2) | 97 (72.4) | .001 |
| Logistic EuroSCORE | 14.8 [10.0-22.0] | 11.0 [7.0-17.0] | 13.6 [10.0-18.0] | 19.8 [14.0-29.0] | .001 |
| SYNTAX score | 0 [0-4] | 0 [0-2] | 0 [0-1] | 2 [0-8] | .001 |
| PCI before TAVI | 35 (8.7) | 8 (6.0) | 5 (3.7) | 22 (16.4) | .001 |
| SYNTAX score II CABG | 41.6 [37.9-48.1] | 40.0 [34.0-44.0] | 40.0 [37.0-47.0] | 45.8 [41.0-53.1] | .001 |
| SYNTAX score II PCI | 40.8 [35.4-45.7] | 33.0 [30.0-35.4] | 40.8 [39.1-42.9] | 48.0 [45.7-52.0] | .001 |
| Echocardiographic characteristics | |||||
| Left ventricular ejection fraction, % | 56.7±18.7 | 58.7±16.2 | 60.8±17.5 | 50.5±20.6 | .001 |
| Mean aortic gradient, mmHg | 47.7±15.7 | 43.7±14.5 | 50.9±15.3 | 48.3±16.6 | .001 |
| Aortic valve area, cm2 | 0.60 [0.50-0.70] | 0.60 [0.50-0.70] | 0.60 [0.40-0.70] | 0.60 [0.50-0.70] | .144 |
| Moderate to severe MR | 33 (8.2) | 10 (7.5) | 11 (8.2) | 12 (9.0) | .906 |
| Moderate to severe AR | 55 (13.8) | 17 (12.8) | 17 (12.8) | 21 (15.8) | .647 |
AR, aortic regurgitation; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MR, mitral regurgitation; PCI, percutaneous coronary intervention; SS-II, SYNTAX score II; TAVI, transcatheter aortic valve implantation.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
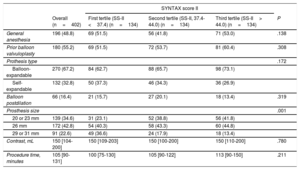
All baseline clinical characteristics included in the SS-II were more frequent in the 3rd SS-II tertile (P <.001), except chronic pulmonary disease (P <.007), which was more frequent in patients in the 1st tertile. Consequently, patients with a higher SS-II were older, more commonly female, had more peripheral vascular disease, lower renal and left ventricular function and less complete revascularization prior to TAVI (higher residual SS-I) (P <.001 for all comparisons). Other clinical variables not included in the SS-II, including diabetes, atrial fibrillation, previous stroke, and hypertension were similarly distributed across SS-II tertiles. Logistic EuroSCORE (1st tertile, 13.58±8.4; 2nd tertile, 15.84±8.99; 3rd tertile, 22.05±10.59; P <.001) was higher with increasing SS-II. Procedural characteristics were similar among all groups (Table 2).
Procedural Characteristics According to SYNTAX Score II Tertiles
| SYNTAX score II | |||||
|---|---|---|---|---|---|
| Overall (n=402) | First tertile (SS-II <37.4) (n=134) | Second tertile (SS-II, 37.4-44.0) (n=134) | Third tertile (SS-II> 44.0) (n=134) | P | |
| General anesthesia | 196 (48.8) | 69 (51.5) | 56 (41.8) | 71 (53.0) | .138 |
| Prior balloon valvuloplasty | 180 (55.2) | 69 (51.5) | 72 (53.7) | 81 (60.4) | .308 |
| Prothesis type | .172 | ||||
| Balloon-expandable | 270 (67.2) | 84 (62.7) | 88 (65.7) | 98 (73.1) | |
| Self-expandable | 132 (32.8) | 50 (37.3) | 46 (34.3) | 36 (26.9) | |
| Balloon postdilation | 66 (16.4) | 21 (15.7) | 27 (20.1) | 18 (13.4) | .319 |
| Prosthesis size | .001 | ||||
| 20 or 23 mm | 139 (34.6) | 31 (23.1) | 52 (38.8) | 56 (41.8) | |
| 26 mm | 172 (42.8) | 54 (40.3) | 58 (43.3) | 60 (44.8) | |
| 29 or 31 mm | 91 (22.6) | 49 (36.6) | 24 (17.9) | 18 (13.4) | |
| Contrast, mL | 150 [104-200] | 150 [109-203] | 150 [100-200] | 150 [110-200] | .780 |
| Procedure time, minutes | 105 [90-131] | 100 [75-130] | 105 [90-122] | 113 [90-150] | .211 |
SS-II, SYNTAX score II.
Data are expressed as No. (%) or median [interquartile range].
In-hospital clinical outcomes after TAVI are reported in Table 3. Increased SS-II was associated with higher 30-day mortality (1st tertile, 5 [3.7%]; 2nd tertile, 7 [5.2%]; 3rd tertile: 15 [11.2%]; P=.036) and more life-threatening or major bleeding complications (P=.015). There was a trend toward increased acute kidney injury in the highest tertile (1st tertile, 30 [23.3]; 2nd tertile, 25 [19.5]; 3rd tertile, 40 [32.5]; P=.051). The presence of CAD alone had no impact on the rate of the combined primary endpoint or on all-cause mortality at 30 days and 1 year.
In-hospital Clinical Outcomes According to SYNTAX Score II Tertiles
| SYNTAX score II | |||||
|---|---|---|---|---|---|
| Overall (n=402) | First tertile (SS-II <37.4) | Second tertile (SS-II, 37.4-44.0) | Third tertile (SS-II> 44.0) | P | |
| (n=134) | (n=134) | (n=134) | |||
| 30-day mortality | 27 (6.7) | 5 (3.7) | 7 (5.2) | 15 (11.2) | .036 |
| Stroke | 9 (2.2) | 4 (3.0) | 3 (2.2) | 2 (1.5) | .711 |
| Myocardial injury | |||||
| Increase troponin × 35 ULN (ULN 0.05 ng/dL) | 149 (39.9) | 44 (35.5) | 51 (41.8) | 54 (42.5) | .459 |
| Increase creatinine kinase-isoenzyme MB × 5 ULN (ULN 5 ng/dL) | 30 (8.4) | 6 (5.0) | 10 (8.5) | 14 (11.6) | .190 |
| Vascular complications | |||||
| Major | 46 (11.5) | 15 (11.3) | 14 (10.5) | 17 (12.7) | .854 |
| Minor | 94 (23.6) | 34 (25.6) | 29 (21.8) | 31 (23.3) | .768 |
| Bleeding complications | |||||
| Life-threatening or major | 65 (16.5) | 17 (12.9) | 16 (12.4) | 32 (24.1) | .015 |
| Minor | 64 (16.2) | 24 (18.0) | 19 (14.6) | 21 (15.8) | .744 |
| Acute kidney injury | |||||
| Stage 1, 2 and 3 | 95 (25.0) | 30 (23.3) | 25 (19.5) | 40 (32.5) | .051 |
| Stage 2 and 3 | 16 (4.2) | 4 (3.1) | 5 (3.9) | 7 (5.7) | .579 |
| New permanent pacemaker replacement | 60 (15.1) | 20 (15.3) | 21 (15.8) | 19 (14.3) | .941 |
| Need for a second valve | 13 (3.2) | 4 (3.0) | 7 (5.2) | 2 (1.5) | .221 |
| Significant aortic regurgitation | 24 (6.5) | 8 (6.2) | 11 (8.9) | 5 (4.3) | .349 |
| Length of CCU stay, d | 1 [1-2] | 1 [1-2] | 1 [1-2] | 1 [1-3] | .816 |
| Length of hospital stay, d | 6 [5-9] | 6 [5-7] | 6 [4-7] | 6 [5-7] | .678 |
| Treatment at hospital discharge* | .537 | ||||
| None | 7 (1.9) | 4 (3.1) | 1 (0.8) | 2 (1.7) | |
| Single antiplatelet therapy | 24 (6.4) | 4 (3.1) | 10 (7.9) | 10 (8.4) | |
| Dual antiplatelet therapy | 207 (55.5) | 67 (52.3) | 71 (56.3) | 69 (58) | |
| Single anticoagulation therapy | 55 (14.7) | 21 (16.4) | 17 (13.5) | 17 (14.3) | |
| Single antiplatelet+anticoagulation therapy | 68 (18.2) | 29 (22.7) | 22 (17.5) | 17 (14.3) | |
| Triple therapy | 12 (3.2) | 3 (2.3) | 5 (4.0) | 4 (3.4) | |
CCU, coronary care unit; SS-II, SYNTAX score II; ULN, upper limit normal.
Values are expressed as No. (%) or median [interquartile range].
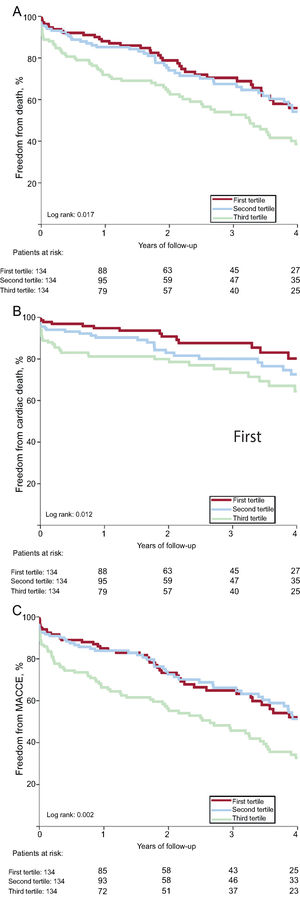
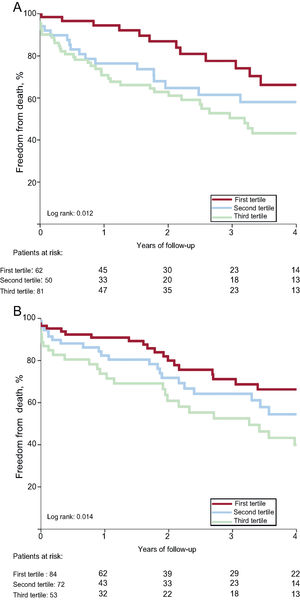
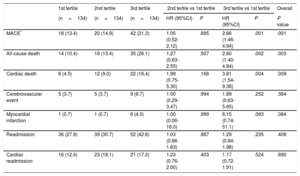
As shown in Table 4, the 1-year risk of major adverse cardiovascular events, all-cause death, and cardiac death was higher among patients in the 3rd SS-II tertile (HR, 2.66; 95%CI, 1.46-4.84; P <.001; HR, 2.60; 95%CI, 1.40-4.84; P=.002, HR, 3.81; 95%CI, 1.54-9.38; P=.004) and was similar among patients in the 2nd tertile (HR, 1.05; 95%CI, 0.52-2-12; P=.895; HR, 1.27; 95%CI, 0.63-2.55; P=.507; HR, 1.99; 95%CI, 0.75-5.30; P=.168) compared with patients in the 1st tertile. There was no significant difference in risk of readmission or cardiac readmission among the groups. At 4 years, a higher SS-II was associated with higher rates of all-cause mortality (P=.017), cardiac mortality (P=.012), and major adverse cardiovascular events (P=.002) (Figure 1). On multivariable analysis, the 3rd SS-II tertile was an independent predictor of 4-year mortality and major adverse cardiovascular events (Table 5). When the population was stratified into patients with and without CAD, the highest SS-II tertile continued to be associated with long-term mortality (Figure 2).
One-year Clinical Outcomes According to SYNTAX Score II Tertiles
| 1st tertile | 2nd tertile | 3rd tertile | 2nd tertile vs 1st tertile | 3rd tertile vs 1st tertile | Overall | |||
|---|---|---|---|---|---|---|---|---|
| (n=134) | (n=134) | (n=134) | HR (95%CI) | P | HR (95%CI) | P | P value | |
| MACE* | 18 (13.4) | 20 (14.9) | 42 (31.3) | 1.05 (0.52-2.12) | .895 | 2.66 (1.46-4.84) | .001 | .001 |
| All-cause death | 14 (10.4) | 18 (13.4) | 35 (26.1) | 1.27 (0.63-2.55) | .507 | 2.60 (1.40-4.84) | .002 | .003 |
| Cardiac death | 6 (4.5) | 12 (9.0) | 22 (16.4) | 1.99 (0.75-5.30) | .168 | 3.81 (1.54-9.38) | .004 | .009 |
| Cerebrovascular event | 5 (3.7) | 5 (3.7) | 9 (6.7) | 1.00 (0.29-3.47) | .994 | 1.89 (0.63-5.65) | .252 | .384 |
| Myocardial infarction | 1 (0.7) | 1 (0.7) | 6 (4.5) | 1.00 (0.06-16.0) | .999 | 6.15 (0.74-51.1) | .093 | .084 |
| Readmission | 36 (27.9) | 39 (30.7) | 52 (42.6) | 1.03 (0.66-1.63) | .887 | 1.29 (0.84-1.98) | .235 | .408 |
| Cardiac readmission | 16 (12.4) | 23 (18.1) | 21 (17.2) | 1.23 (0.76-2.00) | .403 | 1.17 (0.72-1.91) | .524 | .690 |
95%CI, 95% confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular events.
Unless otherwise indicated, data are expressed as No. (%).
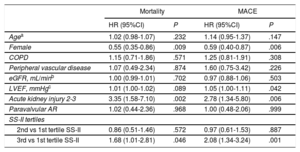
Multivariate Predictors of 4-year All-cause Mortality and Major Adverse Cardiovascular Events After Transcatheter Aortic Valve Implantation
| Mortality | MACE | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Agea | 1.02 (0.98-1.07) | .232 | 1.14 (0.95-1.37) | .147 |
| Female | 0.55 (0.35-0.86) | .009 | 0.59 (0.40-0.87) | .006 |
| COPD | 1.15 (0.71-1.86) | .571 | 1.25 (0.81-1.91) | .308 |
| Peripheral vascular disease | 1.07 (0.49-2.34) | .874 | 1.60 (0.75-3.42) | .226 |
| eGFR, mL/minb | 1.00 (0.99-1.01) | .702 | 0.97 (0.88-1.06) | .503 |
| LVEF, mmHgc | 1.01 (1.00-1.02) | .089 | 1.05 (1.00-1.11) | .042 |
| Acute kidney injury 2-3 | 3.35 (1.58-7.10) | .002 | 2.78 (1.34-5.80) | .006 |
| Paravalvular AR | 1.02 (0.44-2.36) | .968 | 1.00 (0.48-2.06) | .999 |
| SS-II tertiles | ||||
| 2nd vs 1st tertile SS-II | 0.86 (0.51-1.46) | .572 | 0.97 (0.61-1.53) | .887 |
| 3rd vs 1st tertile SS-II | 1.68 (1.01-2.81) | .046 | 2.08 (1.34-3.24) | .001 |
95%CI, 95% confidence interval; AR, aortic regurgitation; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; SS-II, SYNTAX score II.
This study analyzed for the first time the impact of the SS-II on clinical outcomes among patients with severe symptomatic aortic stenosis undergoing TAVI. SYNTAX score II, which adds clinical variables to CAD severity quantified by the angiographic SS-I, was superior to the SS-I in predicting 30-day and 1-year mortality in patients with aortic stenosis undergoing TAVI. Increasing SS-II was associated with increased major adverse cardiovascular events, all-cause death, and cardiac death in the TAVI population independently of the presence of CAD. Patients with an SS-II> 44 had an independent ∼2-fold increased risk of major adverse cardiovascular events and all-cause mortality at 1 year and 4 years compared with patients with an SS-II <37.4.
The presence of CAD and the need for concomitant CABG in patients undergoing surgical aortic valve replacement has been associated with increased rates of periprocedural myocardial infarction and early postoperative mortality.22,23 Concomitant CAD in the typically elderly TAVI population is frequent, at up to 60%.6,24–27 However in this scenario, the evidence demonstrating the influence of pre-existing CAD on procedural outcomes and mid-term survival after TAVI is inconclusive.24,26 While some studies have reported a significant impact on mortality,27,28 others have found no effects on outcomes.7,25,29 A meta-analysis of 7 studies including 2472 patients showed that the presence of CAD does not increase the risk of death (OR, 1.0; 95%CI, 0.67-1.5).30 The anatomic SS-I has been demonstrated to be a useful tool to predict clinical outcomes in patients undergoing revascularization and several randomized trials for both PCI and TAVI are based on or included SS-I in their algorithms,8,30 including PARTNER-231 and SURTAVI.32 A more detailed description of the complexity of CAD in TAVI patients, rather than dichotomous CAD status, has been reported using the SS-I.6,7 While in 1 single-center study, SS-I> 9 was shown to be predictive of mortality in patients undergoing TAVI with the Edwards Sapiens valve,7 Stefanini et al.6 demonstrated that patients with an SS-I> 22 had a higher risk of cardiovascular death, stroke, and myocardial infarction. Furthermore the extent and complexity of CAD assessed by SS-I was associated with baseline risk profiles in both TAVI and surgical aortic valve replacement populations.6,31 The SS-II was developed by combining anatomical variables (anatomic SS-I and the presence of unprotected left main CAD) with clinical variables: age, creatinine clearance, left ventricular ejection fraction, peripheral vascular disease, female sex, and chronic obstructive pulmonary disease. Further validation in the DELTA32 and CREDO-Kyoto33 registries showed that the SS-II can better guide decision-making than the original SS-I in patients with CAD. Given that the presence of CAD is only 1 component of the SS-II and CAD and aortic stenosis have a similar pathophysiology,1,2 it would appear that the SS-II may prove a useful tool in predicting outcomes even in patients without CAD. In our study, we showed an additional predictive value of SS-II, compared with the SS-I and residual SS-I, on 30-day and 1-year mortality in patients undergoing TAVI. This may be explained by the fact that all of the clinical variables included in the SS-II are important factors related to outcomes after TAVI,34,35 and they have more impact on outcomes than the presence or absence of CAD. Poor renal function and left ventricular ejection fraction, age, chronic obstructive pulmonary disease, and peripheral vascular disease have been associated with worse outcomes in the TAVI population. Female sex is the only contradictory variable, being a protective factor in both the SS-II CABG and TAVI studies (“female paradox”) and a predictor of increased risk in the SS-II PCI. It appears that, in this elderly population, comorbidities may play a more important role in clinical outcome than the extent of CAD. When endpoints were assessed in patients with and without CAD using the SS-II, there were no significant differences between the groups, indicating that the SS-II can be used in the entire TAVI population independent of CAD status. Future studies with the expansion of TAVI to lower risk profile patients will determine whether the extent and complexity of CAD plays a more predominant role in clinical outcomes in this setting.
The SS-II was developed to aid in the decision-making process for coronary revascularization in patients with multivessel CAD, and thus generates a probability of mortality for PCI and CABG. In our study, SS-II PCI better predicted 30-day and 1-year mortality. Further analysis will need demonstrate whether this effect continues over a longer period of follow-up. In the present study, SS-II PCI provided a useful stratification of TAVI patients in predicting short-term complications. Importantly, the 3rd tertile had significantly high 30-day mortality, bleeding and acute kidney injury rates (∼12%, 24%, and 32%, respectively), which may classify these patients in the group for whom the intervention is considered futile. This provides an additional tool when discussing the risk and benefits of the intervention. In addition, the SS-II PCI differentiated patients in the highest tertile with a higher risk of 4-year major adverse cardiovascular events and all-cause and cardiovascular death. This stratification continued to be relevant after adjustment for other significant baseline differences and known predictive factors of mortality in the TAVI population.
Several surgical risk scores (especially the Society of Thoracic Surgeons score and EuroSCORE) are used in daily clinical practice for risk assessment in TAVI candidates.36 Although used as part of the Heart Team discussion, they are suboptimal for the assessment of high-risk valvular disease patients.37–39 New TAVI-specific risk scores have also been developed with variable limitations and prognostic capacity40–43 and less penetration than traditional surgical risk scores in current practice.36 Thus, the optimal score for TAVI patients has not yet been developed. In the present study, the predictive ability of the SS-II PCI for 30-day and 1-year mortality was an AUC of 0.632 and 0.619, respectively (P <.05). Silaschi et al.38 reported a nonsignificant AUC for several surgical risk factors in a cohort of 457 patients undergoing TAVI. Although in our study, the association was statistically significant, the predictive value continued to be modest and further efforts should be made to improve clinical risk assessment in this special population. In addition, the impact and management of CAD in patients undergoing TAVI should be addressed in randomized clinical trials. Meanwhile, clinical assessment, risk prediction, and the revascularization strategy before TAVI should be individualized in each patient. The SS-II is an additional, readily available, and useful tool to predict clinical outcomes in patients with aortic stenosis undergoing TAVI.
LimitationsThis is a single-center, observational study and indluded only the transfemoral approach. Therefore, our results need to be confirmed by future larger multicenter studies and with different approaches. However, calculation of the SS-II in a real-world cohort may extrapolate the results to other cohorts. Coronary revascularization was performed according to the Heart Team decision, so it may introduce bias into the analysis and results. The SS-II was developed in an entirely different patient population to those undergoing TAVI and was not specifically designed to assess outcomes in this scenario, which limits the analysis. However, most the risk scores currently used are not designed for TAVI candidates.
CONCLUSIONsThe SS-II PCI appears to be a useful tool in predicting outcomes post-TAVI. Increased SS-II was associated with poorer clinical outcomes at 1 and 4 years post-TAVI. Patients with an SS-II> 44 have a higher risk of cardiovascular death, stroke, or MI than patients with an SS-II <44, independently of the presence of CAD.
CONFLICTS OF INTERESTL. Nombela-Franco has served as a proctor for Abbott.
- –
Aortic stenosis frequently coexists with CAD, and surgical aortic valve replacement in combination with CABG increases periprocedural mortality and morbidity. The impact of CAD in patients undergoing TAVI remains inconclusive. The anatomic SYNTAX score has been investigated as a potential tool to predict major cardiovascular events in patients undergoing TAVI, but its predictive value was found to be very limited
- –
The SS-II, which adds clinical variables to CAD severity quantified by the angiography, is superior to the SYNTAX score in predicting 30-day and 1-year mortality in patients with symptomatic aortic stenosis undergoing TAVI. Increasing SS-II was associated with increased major adverse cardiovascular events, all-cause death, and cardiac death in the TAVI population independently of the presence of CAD. Patients with an SS-II> 44 had an independent ∼2-fold increased risk of major adverse cardiovascular events and all-cause mortality at 1 year and 4 years compared with patients with a SYNTAX score II <37.4. SYNTAX score II is a readily available and easily calculable score, which may be a useful addition to risk assessment in patients undergoing TAVI.