There are scarce clinical outcomes data on the new generation recapturable and repositionable CoreValve Evolut R.
MethodsData on all-comer patients undergoing transcatheter aortic valve implantation (TAVI) with the Evolut R for severe symptomatic aortic stenosis at a single center were prospectively collected between February 2015 and April 2016. Clinical endpoints were independently adjudicated according to the Valve Academic Research Consortium-2 criteria. Primary outcomes consisted of early safety composite endpoints and 30-day device success. The incidence of new permanent pacemaker implantation was recorded.
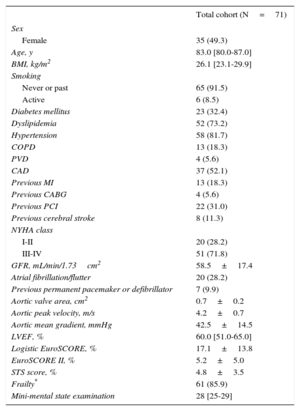
ResultsAmong the 83 patients undergoing TAVI during this period, 71 (85.5% of the population; median age, 83.0 [interquartile range, 80.0-87.0] years; Society of Thoracic Surgeons scores, 4.8±3.5%) were suitable for Evolut R implantation and were included in the analysis. Repositioning was performed in 26.8% of the procedures. The early safety composite endpoint was observed in 11.3% of patients at 30 days, with 2.8% all-cause mortality. Device success was documented in 90.1% of patients. Paravalvular leakage was less than grade II in 98.4% of patients. The mean transvalvular aortic gradient was reduced from 42.5±14.5mmHg at baseline to 7.7±4.0mmHg at discharge (P<.0001 vs baseline). New permanent pacemaker implantation was required in 23.9% of patients.
ConclusionsThe new generation Evolut R is suitable for most patients and shows high device success and acceptable mortality in an unbiased, consecutive, all-comer population at a single center performing TAVI exclusively with Medtronic valves.
Keywords
Over the past decade, transcatheter heart valve (THV) technology has undergone major improvements. However, shortcomings have been identified, despite favorable clinical outcomes in randomized controlled trials against surgery.1,2 Indeed, in addition to providing very long-term durability data, all efforts should be made to reduce vascular access complications, paravalvular leak (PVL), stroke, and permanent pacemaker implantation before expanding the indication for transcatheter aortic valve implantation (TAVI) to lower risk and younger populations. To improve the clinical outcomes and safety profile of the self-expandable CoreValve (Medtronic Inc, Minneapolis, Minnesota, United States), a new generation recapturable and repositionable self-expanding THV has been developed (CoreValve Evolut R system, henceforth referred to as Evolut R). This THV was approved by the Conformité Européenne in September 2014 for the 23-mm size and in February 2015 for the 26-mm and 29-mm sizes and by the Food and Drug Administration in June 2015. Currently, the 31-mm device still only exists in the fourth generation CoreValve model. The purpose of the present study was to report prospectively collected safety data and independently adjudicated clinical outcomes of consecutive patients treated with the Evolut R at a single center using the Medtronic THV exclusively during that period.
METHODSPatient Population and AssessmentFrom the launch of the Evolut R in Switzerland in February 2015 until April 2016, out of the 83 patients who underwent TAVI at a single center, 71 (85.5%) received the Evolut R, while 12 (14.5%) patients received the fourth-generation 31-mm CoreValve due to a large aortic annulus (perimeter between 81.7mm and 91.1mm corresponding to a mean diameter from 26 to 29mm) and were therefore excluded from the analysis. All patients were treated for severe symptomatic aortic stenosis, defined by resting transthoracic echocardiography as an aortic valve area < 1.0cm2 or aortic valve index < 0.6cm2/m2. The logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE), the EuroSCORE II and the Society of Thoracic Surgeons risk score were calculated as part of the operability assessment. Additionally, all patients were assessed by the local multidisciplinary Heart Team composed of interventional cardiologists, cardiac surgeons, cardiovascular anesthesiologists, and intensive care specialists. As a general rule, the Heart Team opted for TAVI in patients with an estimated increased risk for conventional surgery and/or frailty. Patients were considered as frail when 1 of the following criteria was fulfilled: slow gait speed test (< 6seconds), poor grip strength measurement, a history of recent falls, recent weight loss (> 5kg/y), low serum albumin level (< 35g/L), or body mass index (< 20kg/m2). A baseline assessment of medical history, physical examination, transthoracic echocardiography, and biological screening was completed for each patient. Patients gave written informed consent for the TAVI procedure and for the use of related anonymous data for research and publication.
Multislice computed tomography was performed to measure aortic annulus and root dimensions as well as calcifications and to assess minimal iliofemoral or subclavian vascular diameter, tortuosity, and calcifications. Exceptionally, in cases of severe renal insufficiency, magnetic resonance imaging and/or transoesophageal echocardiography were performed to measure the aortic annulus and root dimensions. An additional vascular assessment with an abdominal aortography including iliofemoral vessels and selective femoral angiographies was performed at the time of the coronary angiogram at the operator's discretion for patients with available multislice computed tomography and in all patients without multislice computed tomography.
DeviceThe Evolut R is a radiopaque self-expanding nitinol support frame with a porcine pericardium skirt and supra-annular trileaflet pericardial valve, which is treated with alpha-amino oleic acid. The cell geometry and frame of the prosthesis has been redesigned: the objective was to improve conformability to the aortic annulus in order to reduce PVL and to optimize frame interaction with the native annulus. In addition, radial force is more homogenous across the different device sizes at the level of the inflow, and the outflow has been shortened with a paddle design instead of the previously used tabs to facilitate valve release from the delivery catheter. The EnVeo R delivery catheter has an InLine sheath (a 14-Fr equivalent, corresponding to a true 18-Fr outer diameter), which allows the valve to be delivered without the requirement of a separate introducer sheath. The novel laser-cut nitinol-reinforced capsule is designed to make resheathing or recapture of the valve possible up to the point of 80% of maximal valve deployment (considered as the “point of no return”).
ProcedureVascular access was commonly obtained by percutaneous artery puncture, while a surgical cutdown was preferred for selected iliofemoral anatomies. Once arterial access was achieved, angiography was performed to ensure the correct entry site of the sheath at the level of the common femoral artery. For the percutaneous approach, preclosure was performed using the Prostar XL 10 suture-based vascular closure device (Abbott Vascular, Reedwood City, California, United States). In cases of femoral access-limiting peripheral arterial disease, a balloon expandable sheath (Solopath Onset Medical, a subdivision of Terumo Medical Corporation, Irvine, California, United States) was considered. Native aortic valve predilatation was performed if there was heavy leaflet calcification. Angiography of the aortic root was performed before, during and after valve deployment to assess prosthesis position and functionality. No transesophageal echocardiographic guidance was routinely used during the procedure. Access closure was performed using the Prostar XL 10 under no-flow conditions following inflation of a peripheral balloon in the external iliac or common iliac artery using crossover contralateral femoral access. Complete hemostasis and the absence of vascular complications were confirmed by crossover angiography. The antithrombotic regimen consisted of dual antiplatelet therapy with aspirin and clopidogrel for 3 months followed by aspirin monotherapy, when no oral anticoagulation was required. In patients with chronic anticoagulation, aspirin monotherapy was added for 3 months.
Study EndpointsAll clinical endpoints were independently adjudicated according to the Valve Academic Research Consortium (VARC)-2 criteria.3 Primary outcomes consisted of the VARC-2 early safety composite endpoints and device success at 30 days. Early safety composite endpoints included the incidence of all-cause mortality, all strokes, life-threatening bleeding, acute kidney injury, coronary obstruction requiring intervention, major vascular complications, and valve-related dysfunction requiring a repeat procedure. Valve Academic Research Consortium-2 device success is defined as the absence of procedural mortality, correct positioning of a single THV and intended performance of the prosthesis. Independent interventional cardiologists from the Swiss TAVI registry performed endpoint adjudication. The incidence of new pacemaker implantation was also recorded.
Follow-up and Data CollectionA clinical assessment, 12-lead electrocardiogram, and transthoracic echocardiography were performed before hospital discharge and as part of the 30-day follow-up either in our institution or by the patient's private cardiologist. When collected from a private practice, documentation was obtained from the treating physician. All data up to the 30-day follow-up visit were collected as part of our local prospective registry, which was approved by the local ethics committee, and were also entered in the Swiss TAVI registry.
Statistical AnalysisCategorical data are presented as numbers and percentages. Normally distributed continuous variables are expressed as mean±standard deviation and nonnormally distributed variables as median [interquartile ranges]. The distribution of continuous variables was assessed by visual inspection of histograms and was confirmed using the Shapiro-Wilk test. The mean aortic gradient at baseline and before discharge was compared using the Student t test and is presented as error bars with standard deviation. Statistical analyses were performed using IBM SPSS Statistics version 23 (IBM, Armonk, New York, United States).
RESULTSPatient PopulationDuring the study period, out of the 83 consecutive patients undergoing TAVI at the institution, 71 (85.5%) were considered suitable for Evolut R with the currently available valve sizes and were included in the analysis. Median age was 83.0 [80.0-87.0] years and 49.3% of the patients were women. Baseline patient characteristics and echocardiographic parameters are presented in Table 1. The mean calculated logistic EuroSCORE was 17.1±13.8%, mean EuroSCORE II was 5.2±5.0%, and mean Society of Thoracic Surgeons score was 4.8±3.5%. Sixteen (22.5%), 7 (9.9%) and 11 (15.5%) patients had a logistic EuroSCORE>20%, EuroSCORE II>10%, and a Society of Thoracic Surgeons score > 8%, respectively. A total of 85.9% of the patients met the criteria for at least 1 frailty measurement. Seven patients (9.9%) already had a permanent pacemaker at the time of the TAVI procedure.
Baseline Characteristics
| Total cohort (N=71) | |
|---|---|
| Sex | |
| Female | 35 (49.3) |
| Age, y | 83.0 [80.0-87.0] |
| BMI, kg/m2 | 26.1 [23.1-29.9] |
| Smoking | |
| Never or past | 65 (91.5) |
| Active | 6 (8.5) |
| Diabetes mellitus | 23 (32.4) |
| Dyslipidemia | 52 (73.2) |
| Hypertension | 58 (81.7) |
| COPD | 13 (18.3) |
| PVD | 4 (5.6) |
| CAD | 37 (52.1) |
| Previous MI | 13 (18.3) |
| Previous CABG | 4 (5.6) |
| Previous PCI | 22 (31.0) |
| Previous cerebral stroke | 8 (11.3) |
| NYHA class | |
| I-II | 20 (28.2) |
| III-IV | 51 (71.8) |
| GFR, mL/min/1.73cm2 | 58.5±17.4 |
| Atrial fibrillation/flutter | 20 (28.2) |
| Previous permanent pacemaker or defibrillator | 7 (9.9) |
| Aortic valve area, cm2 | 0.7±0.2 |
| Aortic peak velocity, m/s | 4.2±0.7 |
| Aortic mean gradient, mmHg | 42.5±14.5 |
| LVEF, % | 60.0 [51.0-65.0] |
| Logistic EuroSCORE, % | 17.1±13.8 |
| EuroSCORE II, % | 5.2±5.0 |
| STS score, % | 4.8±3.5 |
| Frailty* | 61 (85.9) |
| Mini-mental state examination | 28 [25-29] |
BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EuroSCORE, European System for Cardiac Operative Risk Evaluation; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STS, Society of Thoracic Surgeons.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
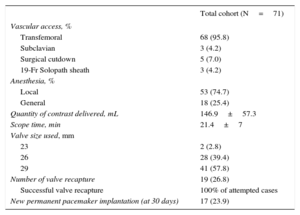
Procedural characteristics are presented in Table 2. A fully percutaneous vascular approach was performed in 93.0% and the remaining patients (7.0%) underwent surgical femoral cutdown. The EnVeo R delivery catheter was advanced without an additional sheath in 68 (95.8%) patients, and a balloon expandable sheath, namely the 19-Fr Solopath sheath (Terumo, Japan), was used in 3 (4.2%) patients due to iliofemoral disease and borderline minimal diameter. In 36 (50.7%) patients, predilatation before valve implantation was performed and 23 (32.4%) patients underwent postdilatation of the prosthesis during the same index procedure. For 25.0% (9 of 36) of the patients who underwent predilatation, postdilatation was also required. The 29-mm valve size was the most frequently implanted prosthesis (57.8% of patients). Repositioning was attempted in 17 patients (23.9% of patients) by resheathing the prosthesis and was invariably successful. No recapture with full prosthesis removal was performed. Valve embolization requiring immediate surgical conversion occurred in 1 patient (1.4%) due to a technical error: indeed, while the prosthesis was not completely deployed, but was beyond the point of no return in the deployment process, the paddle on the inner curve of the aorta remained partially covered by the nonradio-opaque tip of the capsule and was consequently still attached to the delivery system (Figure 1). Therefore, when the delivery catheter was withdrawn, it pulled the prosthesis into the ascending aorta. Since the valve was floating in the ascending aorta and the patient was hemodynamically stable and had an intermediate surgical risk, conversion to surgery in the operating room was proposed to remove the prosthesis, and the patient concurrently underwent surgical aortic valve replacement. Postoperative recovery was complicated by a urinary tract infection resulting in bacteremia and new onset of atrial fibrillation. Three patients (4.2%) required implantation of a second valve during the same index procedure due to a too-high valve implantation with significant PVL. In each of these 3 patients, the first valve prosthesis was implanted at the upper limit and migrated slightly above this limit during the full valve release beyond the point of no return of the resheathing capsule.
Procedural Outcomes
| Total cohort (N=71) | |
|---|---|
| Vascular access, % | |
| Transfemoral | 68 (95.8) |
| Subclavian | 3 (4.2) |
| Surgical cutdown | 5 (7.0) |
| 19-Fr Solopath sheath | 3 (4.2) |
| Anesthesia, % | |
| Local | 53 (74.7) |
| General | 18 (25.4) |
| Quantity of contrast delivered, mL | 146.9±57.3 |
| Scope time, min | 21.4±7 |
| Valve size used, mm | |
| 23 | 2 (2.8) |
| 26 | 28 (39.4) |
| 29 | 41 (57.8) |
| Number of valve recapture | 19 (26.8) |
| Successful valve recapture | 100% of attempted cases |
| New permanent pacemaker implantation (at 30 days) | 17 (23.9) |
Values are expressed as No. (%) or mean±standard deviation.
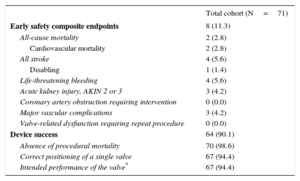
At 30 days, the early safety composite endpoint was observed in 8 (11.3%) patients, while 2 patients (2.8%) died. The first died of sudden death 6 days after the TAVI procedure due to a malignant ventricular arrhythmia, which was probably related to cardiac amyloidosis that was first diagnosed at autopsy. The patient had a permanent pacemaker implanted at day 2 post-TAVI and the transthoracic echocardiography performed the day before death showed normal systolic and diastolic function with no valve dysfunction or pericardial effusion. In the second patient, severe renal bleeding was diagnosed 12hours after TAVI, which was stopped by coil embolization. However, the patient died on day 4 from multiorgan failure. With respect to the cause of the perforation, the hydrophilic 0.35” wire used for vascular access closure may have been inadvertently advanced in the kidney vasculature, causing a perforation. Alternatively, the bleeding may have been related to dual antiplatelet therapy and periprocedural anticoagulation. This death was considered procedure-related. Three other cases of nonfatal life-threatening bleeding were reported, including 2 access site-related bleedings and 1 as a consequence of lysis for stroke after the index hospitalization. The first access-site bleeding was retroperitoneal and was related to a wire perforation of a small branch of the internal iliac artery, which occurred at the time of the crossover performed to check vascular closure. It was not seen on the final angiogram, but a computed tomography scan at day 5 performed for back pain and a drop in hemoglobin showed a retroperitoneal hematoma with active bleeding that required coil embolization of the distal branch. The second access-site life-threatening bleeding was due to a closure device failure requiring unplanned endovascular stenting of the right common femoral artery. Three patients (4.2%) experienced VARC-2 major vascular complications related to vascular perforation or access-site closure device failure, which are described above.
Four patients (5.6%) had strokes, including 1 disabling stroke (1.4%) (Rankin score of 4). In 1 patient, a nondisabling stroke was identified at 10 days following TAVI on the basis of a cerebral multislice computed tomography conducted for acute confusion. The remaining VARC-2 early safety composite endpoints are presented in Table 3.
Valve Academic Research Consortium-2 Early Safety Composite Endpoints (at 30 Days) and Device Success
| Total cohort (N=71) | |
|---|---|
| Early safety composite endpoints | 8 (11.3) |
| All-cause mortality | 2 (2.8) |
| Cardiovascular mortality | 2 (2.8) |
| All stroke | 4 (5.6) |
| Disabling | 1 (1.4) |
| Life-threatening bleeding | 4 (5.6) |
| Acute kidney injury, AKIN 2 or 3 | 3 (4.2) |
| Coronary artery obstruction requiring intervention | 0 (0.0) |
| Major vascular complications | 3 (4.2) |
| Valve-related dysfunction requiring repeat procedure | 0 (0.0) |
| Device success | 64 (90.1) |
| Absence of procedural mortality | 70 (98.6) |
| Correct positioning of a single valve | 67 (94.4) |
| Intended performance of the valve* | 67 (94.4) |
AKIN, Acute Kidney Injury Network.
Values are expressed as No. (%).
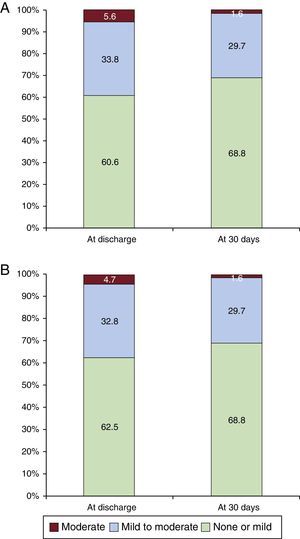
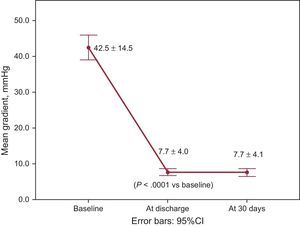
The overall VARC-2 device success rate was 90.1% with only 1 case of procedural death. The PVL was classified as less than moderate in 94.4% and 98.5% of patients, and moderate in 5.6% and 1.6%, at discharge and 30 days, respectively. No severe PVL was observed (Figure 2A). Figure 2B shows the results of PVL assessment, excluding the 7 (9.9%) patients without 30-day echocardiographic follow-up at our institution. Among these 7 patients, only 1 had moderate PVL at discharge but with a New York Heart Association functional class I at 30 days. The mean transvalvular aortic gradient was reduced from 42.5±14.5mmHg at baseline to 7.7±4.0mmHg at discharge (P<.0001 vs baseline) and 7.7±4.1mmHg at 30 days (Figure 3).
New permanent pacemaker implantation was required in 17 patients (23.9%; 26.6% excluding patients with a previous pacemaker) and all implantations except 1 occurred during the index hospitalization period. Indications included third-degree atrioventricular block (52.9% of new implantations), alternating left and right bundle branch block (17.7%), first-degree block associated with either right or left bundle branch block (17.7%), and atrial fibrillation with slow ventricular response (11.8%).
DISCUSSIONAt the present time, the only series available on Pubmed for the new generation, recapturable and repositionable self-expanding Evolut R is the CE Mark Clinical Study,4 which involved 60 patients, who were likely selected. Two other series included patients with the Evolut R prosthesis; outcomes were, however, not established to directly assess the safety and efficacy of this new generation device.5,6 The first-in-human experience took place in September 2013 with a 23-mm Evolut R successfully implanted in a degenerated aortic bioprosthetic valve without any need for recapture.7 To the best of our knowledge, our study is the first to report the performance of Evolut R in a real-world setting: a consecutive, all-comer, elderly population with no selection bias, because we performed TAVI exclusively with the Evolut R or, when the annulus had a perimeter larger than 81.7mm, with the 31-mm CoreValve. All patients considered at high risk or inoperable by the Heart Team during the study period had an aortic anatomy allowing implantation of 1 of the 2 above-mentioned prosthesis. The main findings of the present study are thus the following: a) The device is suitable for 85.5% of all-comer patients treated by TAVI with the currently available Evolut R valve sizes. b) Implantation was associated with a high device success rate (90.1% of the patients). c) Patient outcomes showed acceptable associated morbidity and mortality (all-cause mortality, 2.8%).
Valve Academic Research Consortium-2 Early Safety Clinical EndpointsAlthough TAVI is nowadays still preferentially performed in patients at high or prohibitive surgical risk, short- and midterm mortality remains acceptable in most published series. We report a mortality rate of 2.8% at 30 days, which is in line with previous randomized and nonrandomized reports of self-expanding valve implantation outcomes1,8–10 and lower mortality rates than those reported by some national registries.11,12 The 2 patients in our series who died were considered inoperable and were older than 85 years old.
In the first randomized trial, PARTNER Cohort A, which compared transcatheter vs surgical valve replacement, the stroke rate was significantly higher among patients with TAVI; however, the opposite was shown in the more recent United States CoreValve randomized trial.1,2 Valve Academic Research Consortium-2 disabling strokes are, however, rare events and seem to occur independently of valve type and vascular access.13 In our series, a disabling stroke occurred in 1 patient (1.4%). With respect to the recapturability properties of the Evolut R, among the 15 patients requiring valve recapture for repositioning, similarly to the CE Mark Clinical Study,4 none experienced a periprocedural stroke. The influence of aortic valvuloplasty before TAVI on stroke incidence has become a matter of debate. Even though Pagnesi et al.5 recently reported no difference in terms of stroke rates and mortality at 1 year between TAVI with or without predilatation, the absence of predilatation was, however, associated with a higher incidence of acute stroke at 30 days. In contrast, postdilatation was previously reported as an independent predictor of acute cerebrovascular events.14 In our study, 50.7% of patients underwent predilatation with an 8.3% (3 of 36 patients) stroke rate at 30 days and 1 patient with a nondisabling stroke had postdilatation. Nonetheless, other factors including new onset atrial fibrillation might also play an important role in stroke incidence in addition to the procedure itself. Indeed, half of the strokes (2 of 4 patients) were diagnosed more than 7 days following the procedure.
Reduction in valve delivery catheter diameters combined with growing operator experience and better preoperative vascular access screening has led to a decrease in vascular complications. In the present report, vascular complications occurred in 15.5% of patients and all could be successfully managed percutaneously, although 1 patient (with life-threatening renal bleeding) finally died of multiple organ failure related to a delay in the diagnosis. Even though vascular complications following TAVI remain frequent, percutaneous management is feasible and safe.15 Stortecky et al.16 reported similar clinical outcomes at 30 days between patients with vascular complications treated percutaneously and patients without vascular complications. However, the distinction between major and minor vascular complications according to VARC-2 is of primordial importance, because only major vascular complications are associated with higher mortality.17 According to VARC-2, we observed fewer major vascular complications (4.2% of patients) compared with the CE Mark Clinical Study with the Evolut R system (8.3%). In comparison with that study, we report fairly similar life-threatening bleeding (5.0% vs 5.6% in our experience) and the small difference is mainly due to 1 case of intracranial bleeding following lysis for stroke 18 days after the TAVI procedure in a patient with atrial fibrillation.
Valve Academic Research Consortium-2 Device SuccessDevice success was achieved in 90.1% of patients and was higher than the success rate reported by Manoharan et al.4 with the Evolut R system (78.6%). Our results are, however, similar to those of several other recent studies using self-expanding THV with VARC-2 device success rates varying from 77.5% to 96%.4,10,18 Paravalvular leak are currently considered as one of the main limitations of the available THV and moderate PVLs to a large extent account for the failure of device success in our cohort. Evidence is provided by a PARTNER trial subanalysis,19 which reported a strong influence of PVL on long-term mortality. This association was recently confirmed by a large meta-analysis.20 The resheathing and recapturing properties of the Evolut R are of particular interest as they aid resolution of PVL following TAVI malposition. Furthermore, the optimized oversizing of the Evolut R enhances native valve cover. Finally, the nitinol frame provides consistent radial force, decreasing PVL during the days following the TAVI procedure, resulting from the continued prosthetic expansion. This was represented in our study by the reduction of moderate PVL from 5.6% to 1.6% of patients at discharge and 30 days, respectively. In total, valve recapture was performed 17 times in 15 patients to optimize prosthesis positioning and all attempts at valve recapture were successful.
New Pacemaker ImplantationNew pacemaker implantation was the most frequent adverse event (23.9% of patients) in the present study. Rates of new pacemaker implantation following TAVI are higher among patients receiving self-expanding vs balloon-expanding valves and vary in the literature from 11.7% to 39.3% at 30 days for the self-expanding device.1,4,10,21–24 Device depth has been reported as a strong independent predictor for the development of conduction abnormalities and the need for permanent pacemaker implantation.22 The CE Mark Clinical Study showed a lower new permanent pacemaker rate (11.7%) than in our early experience with the Evolut R (23.9%). Interestingly, when permanent pacemakers were implanted in the CE Mark Clinical Study, the mean implantation depth with respect to the noncoronary cusp was 8.1±3.5mm, whereas when no permanent pacemaker was required, the mean depth of implantation was 3.3±2.5mm. Therefore, one of the potential explanations for our higher rate of new permanent pacemaker implantation could be that following the migration of the 3 valves implanted at the upper limit, which moved slightly above the annulus during full valve release, we were more conservative in the implantation height.
LimitationsThe main limitation of the study is the small number of patients included, resulting in low statistical power. In addition, PVL assessment was not performed by an independent core laboratory. However, all the predischarge transthoracic echocardiographies were reviewed by a senior echocardiographer not involved with the procedure. On the basis of risk calculators (EuroSCORE or Society of Thoracic Surgeons score) alone, our population could be considered as being at intermediate surgical risk. However, frailty assessment, including gait speed and strength tests, reflects the high vulnerability of these elderly patients.
CONCLUSIONSThe new generation Evolut R system is suitable for most patients and showed high device success and acceptable morbidity and mortality in a consecutive, unbiased, all-comer, elderly population treated in a single center exclusively implanting Medtronic THV. While the reduced sheath size and recapturability have the potential to improve clinical outcomes over first generation devices, this benefit will have to be demonstrated in large multicenter studies.
FUNDINGGEcor (foundation for cardiology research at the Division of Cardiology of the University Hospital of Geneva).
CONFLICTS OF INTERESTS. Noble is a proctor for Medtronic CoreValve and Evolut R. M. Roffi received institutional research grants from Abbott Vascular, Boston Scientific, Biotronik, Biosensor and Medtronic.
- –
TAVI has become the new standard of care for severe symptomatic aortic stenosis in patients at high or prohibitive surgical risk.
- –
PVLs, vascular access complications, strokes and new pacemaker implantations remain major limitations that should be better addressed before considering TAVI in lower risk patients.
- –
To optimize valve implantation accuracy and improve the safety profile of THV, a new generation of repositionable and recapturable self-expanding prosthesis, CoreValve Evolut R (Medtronic Inc, Minneapolis, United States), has been developed.
- –
The new generation Evolut R shows high device success with low all-cause mortality (2.8%) at 30 days.
- –
This new generation of THV is suitable in most patients because all of those considered at high risk or inoperable by the Heart Team during the study period had an aortic anatomy allowing implantation of the Evolut R when the aortic annulus was < 81.7mm (29mm being the largest available Evolut R valve size).
- –
This is the first report of the performance of Evolut R in a real-world setting, consisting of a consecutive and all-comer population.