The present study sought to establish the diagnostic yield of cardiovascular magnetic resonance (CMR) in a large cohort of patients admitted with myocardial infarction (MI) with nonobstructive coronary artery disease (MINOCA) based on the timing of referral to CMR.
MethodsConsecutive patients referred to CMR from January 2009 to February 2022 with a working diagnosis of MINOCA were retrospectively evaluated. Cine, T2-weighted, early, and late gadolinium-enhanced images were acquired and analyzed. The frequency of the underlying diagnosis and the association between timing of CMR and relative frequency of each diagnosis were assessed.
ResultsWe included 207 patients (median age 50 years, 60% men). Final diagnosis after CMR was achieved in 91% of the patients (myocarditis in 45%, MI in 20%, tako-tsubo cardiomyopathy in 19%, and other cardiomyopathies in 7%). The performance of CMR within 7 days of admission with MINOCA (median, 5 days; 117 patients) allowed a higher diagnostic yield compared with CMR performed later (median, 10 days; 88 patients) (96% vs 86%, P=.02). Although myocarditis was the most frequent diagnosis in both groups according to time to CMR, its frequency was higher among patients with a CMR performed within the first 7 days (53% vs 35%, P=.02). The frequency of other underlying diagnoses was not influenced by CMR timing.
ConclusionsCMR led to an underlying diagnosis of MINOCA in 91% of patients and its diagnostic yield increased to 96% when CMR was performed within 7 days of admission. The most frequent diagnosis was myocarditis..
Keywords
Myocardial infarction (MI) with nonobstructive coronary artery diseases (MINOCA) has been reported in 5%-6% of patients presenting with an acute MI.1 Current practice guidelines of the European Society of Cardiology (ESC) define MINOCA as acute MI criteria without coronary artery stenosis ≥ 50% and no other specific alternate diagnosis for the clinical presentation.2 The diagnosis of MINOCA is challenging and is most often based on exclusion of other diagnoses such as obstructive coronary artery stenosis, myocarditis, or tako-tsubo cardiomyopathy. Cardiovascular magnetic resonance (CMR) imaging plays an important role in the differential diagnosis of patients with MINOCA since this imaging technique provides information on the myocardial tissue characteristics, allowing differentiation between myocarditis, tako-tsubo cardiomyopathy and MI once coronary angiography has shown patent coronary arteries. According to several studies, CMR provides a final etiologic diagnosis in 64% to 86% of cases.3–9 The reasons for this variable diagnostic yield lie in the differences in distribution of various pathophysiological mechanisms across the series and in the differences in timing and type of the CMR technique used. Although the latest ESC guidelines encourage the performance of CMR as soon as possible after presentation in patients with MINOCA,2 only a few studies have explored the importance of timing of CMR to optimize diagnostic accuracy. The available evidence suggests that an early CMR (< 14 days from presentation) leads to a higher diagnostic yield compared with a later timing.5–8 In the present study, we evaluated the diagnostic yield of CMR in a large cohort of patients with MINOCA, taking into consideration the timing of CMR imaging.
METHODSStudy populationThis longitudinal observational study included consecutive patients aged 18 years or older with a working diagnosis of MINOCA, as per the ESC definition2 who underwent CMR at the University Hospital Germans Trias i Pujol (Badalona, Spain). The inclusion period was between January 2009 and March 2022 and the patients were identified retrospectively from the institutional CMR database. Patients presenting with heart failure as the main clinical symptom or an electrocardiogram without sinus rhythm at admission were excluded. Other CMR-related exclusion criteria were patients with nonmagnetic resonance-conditional pacemakers or implantable cardioverter-defibrillators, claustrophobia, or severe renal impairment (estimated glomerular filtration rate <30mL/min/1.73 m2).
Myocardial injury was defined as levels of troponin I ≥ 0.5 ng/mL and high-sensitivity troponin I (hsTnI) ≥ 30 pg/mL. A typical rise and fall in levels in serial assessments of these biomarkers were required to define MI.10 During the inclusion period, the assay used to analyze the levels of troponin changed. Therefore, the maximal ratio of troponin calculated as the maximal troponin value at admission divided by the upper limit of the normal value for the troponin assay used is reported. Nonobstructed coronary arteries were defined as <50% stenosis in any epicardial coronary artery,1 either on invasive coronary angiography or on coronary computed tomography angiography.
The study was performed in accordance with the Declaration of Helsinki and was reviewed and approved by the Institutional Review Board (ethics code: PI-22-048 and PI-19-257). Due to the retrospective analysis of clinically acquired data, the local ethics committee waived the need for patient written informed consent.
Clinical dataPatient-related data, including baseline demographics, cardiovascular risk factors and comorbidities as well as electrocardiogram and biomarkers throughout admission were obtained from medical reports. Obesity was defined as a body mass index ≥ 30kg/m2.11 Dyslipidemia was defined as total cholesterol >250mg/dL and/or low-density lipoprotein-cholesterol >130mg/dL12 and diabetes as an HbA1c >6.5% and/or fasting plasma glucose >126mg/dL.13 Previous coronary artery disease included prior history of acute MI and/or percutaneous coronary intervention or coronary artery bypass graft surgery. Renal disease was defined as a reduced glomerular filtration rate (< 90mL/min/1.73 m2) using the CKD-EPI formula.14 Psychiatric disorders included depressive syndrome, anxiety, and personality disorders. Previous use of aspirin and other antiplatelet therapies, anticoagulation treatment, beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was reviewed.
Cardiovascular magnetic resonance data acquisition and analysisCMR imaging was performed on a 1.5 Tesla magnetic resonance imaging scanner (Achieva, Philips, The Netherlands). Time to CMR was defined as the number of days from admission with MINOCA to CMR study. The CMR data acquisition protocol included functional cine imaging using breath-hold balanced steady-state free precession sequences in the 3 long-axis views and in short-axis orientation covering both ventricles from the base to the apex of the left ventricle (8-mm slice thickness and 2-mm slice gap). T2-weighted short-tau inversion-recovery (T2-STIR) images were used to detect myocardial edema and were acquired in the same long- and short-axis views as cine sequences. After 2 to 3minutes of intravenous administration of gadolinium-based contrast agent gadobutrol [0.15 mmol/kg; Gadovist (Bayer-Schering Pharma, Germany)], early gadolinium enhancement images were acquired in the 3 long-axis views to evaluate the presence of hyperemia, microvascular obstruction, or intraventricular thrombus. To assess myocardial necrosis/fibrosis, late gadolinium enhancement (LGE) images acquired 8 to 10minutes after contrast administration with inversion-recovery segmented gradient echo sequences in the same long- and short-axis views as cine sequences were used. Inversion time was adjusted to null healthy myocardium over time. The same standardized protocol was maintained throughout the inclusion period with no significant changes in the sequences performed (supplementary data).
Quantification of ventricular volumes and ejection fraction of both ventricles and left ventricular (LV) mass was performed using the short-axis cine stack as described previously, using the Portal IntelliSpace software (Philips Healthcare, The Netherlands).15 Ventricular volumes and LV mass were indexed using the body surface area calculated with the Dubois formula.16
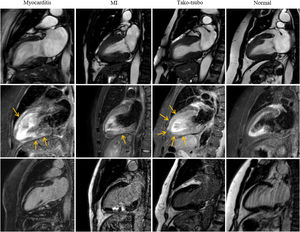
The presence and distribution of regional and global LV wall motion abnormalities using the 17-segment model of the American Heart Association were evaluated.17 In addition, the distribution of myocardial edema identified as high-intensity signal areas in T2-STIR images was analyzed. Finally, the pattern and extent of myocardial LGE (subendocardial, mid-wall, or subepicardial) were evaluated, allowing the classification of the patients into 5 CMR-based diagnostic groups: acute myocarditis, acute MI, tako-tsubo cardiomyopathy, cardiomyopathy group, or normal CMR. Acute myocarditis was defined as the presence of subepicardial and/or transmural edema with patchy mid-wall or subepicardial LGE with or without associated wall motion abnormalities, based on the Lake Louise criteria.18 Acute MI was defined by the presence of regional LV wall motion abnormalities with edema and subendocardial to transmural LGE pattern in the territory supplied by a coronary artery. Tako-tsubo cardiomyopathy was diagnosed by the presence of apical and/or mid-ventricular wall motion abnormality in cine sequences beyond an epicardial vascular distribution with transmural myocardial edema in the same segments and in the absence of significant LGE.19 The cardiomyopathy group included diagnoses of dilated cardiomyopathy, hypertrophic cardiomyopathy, sarcoidosis, or arrhythmogenic right ventricular cardiomyopathy based on CMR criteria.20–22 CMR was considered normal if there were no wall motion abnormalities, myocardial edema or specifical LGE patterns and no other structural or functional abnormalities (figure 1).
The first row shows end-systolic SSFP-cine images in 2-chamber view for each diagnostic. The second row shows T2-weighted images for edema detection (arrows) and third row, T1-weighted images after gadolinium administration to study LGE. In the myocarditis case, there is linear subepicardial edema and LGE in the anterior wall and patchy intramyocardial in the mid-basal inferior wall. The acute myocardial infarction case shows transmural edema in the mid-inferior wall with subendocardial to transmural LGE in the same segment. In the tako-tsubo cardiomyopathy case, there is akinesia and transmural edema in mid-apical segments without LGE. No abnormalities are seen in the normal case. CMR, cardiovascular magnetic resonance; LGE, late gadolinium enhancement; MI, myocardial infarction.
Normal distribution of continuous variables was assessed with the Shapiro-Wilk test. Continuous variables with a normal distribution are presented as mean±standard deviation while nonnormally distributed variables are presented as the median [interquartile range]. Categorical variables are presented as frequencies and percentages. The Student t test or variance analysis were used for comparison of continuous variables. The Fisher or Pearson chi-square tests were used for comparison of dichotomous and categorical variables between groups. Stata software package (version 15.1, StataCorp, College Station, United States) was used for statistical analysis.
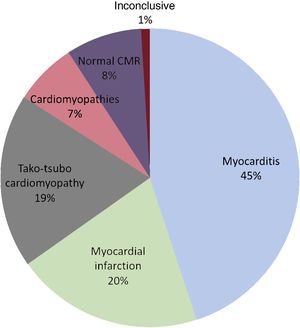
RESULTSWe identified 207 patients with an initial diagnosis of MINOCA and imaged consecutively with CMR. Based on CMR findings, diagnosis was achieved in 91% of patients (188 patients): acute myocarditis in 93 patients (45%), MI in 42 (20%), tako-tsubo cardiomyopathy in 39 (19%), and other cardiomyopathies in 14 (7%), including 7 patients with dilated cardiomyopathy, 5 with hypertrophic cardiomyopathy, 1 patient with arrhythmogenic right ventricular cardiomyopathy, and 1 patient with cardiac sarcoidosis. In 17 patients (8%), CMR demonstrated a structurally normal heart (figure 2). Two patients had an inconclusive CMR: 1 had a final diagnosis of pericarditis and the other patient remained without a final diagnosis at discharge. These 2 patients were excluded from the subsequent analysis. The median time from hospital admission to CMR examination was 7 [5-9] days without significant differences in CMR delay across the diagnostic groups for the whole cohort.
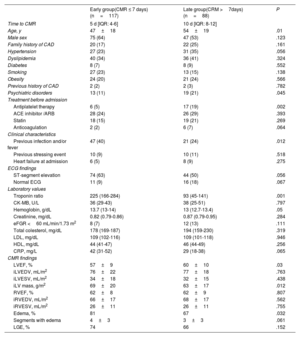
Baseline characteristics and CMR findingsClinical characteristics from the overall cohort and for each diagnostic group are presented in table 1. The mean age of the population was 50±18 years and there was a predominance of men (60%). Almost 90% of patients had an abnormal electrocardiogram at presentation with ST-segment elevation as the most usual finding (118 patients [57%]). Concomitant heart failure symptoms at presentation were observed in 14 patients (7%), while there were no patients with pulmonary edema or cardiogenic shock. Male sex was more frequent in the myocarditis and the normal CMR groups (76% and 71%, respectively) whereas female sex was more frequently observed in the tako-tsubo cardiomyopathy group (79%). Age was youngest in the myocarditis group followed by the MI group, and was oldest in the group of tako-tsubo cardiomyopathy.
Baseline characteristics of the study population
| Total(N=207) | Myocarditis (n=93) | MI(n=42) | Tako-tsubo (n=39) | Cardiomyopathies (n=14) | Normal (n=17) | P | |
|---|---|---|---|---|---|---|---|
| Age, y | 50±18 | 39±16 | 54±14 | 69±12 | 57±14 | 59±15 | <.001 |
| Male sex | 124 (60) | 71 (76) | 23 (55) | 8 (21) | 8 (57) | 12 (71) | <.001 |
| Family history of CAD | 42 (21) | 17 (19) | 13 (31) | 5 (13) | 3 (21) | 4 (25) | .537 |
| Hypertension | 69 (34) | 10 (11) | 16 (38) | 20 (51) | 7 (50) | 5 (29) | <.001 |
| Dyslipidemia | 101 (49) | 26 (28) | 27 (64) | 28 (72) | 9 (64) | 11(65) | <.001 |
| Diabetes | 16 (8) | 3 (3) | 4 (10) | 6 (15) | 6 (43) | 3 (18) | .046 |
| Smoking | 41 (20) | 20 (22) | 10 (24) | 2 (5) | 4 (29) | 4 (24) | .088 |
| Obesity | 45 (22) | 16 (17) | 9 (21) | 8 (21) | 6 (43) | 6 (35) | .301 |
| Previous history of CAD | 4 (1.9) | 0 | 2 (5) | 1 (3) | 0 | 1 (6) | .499 |
| Psychiatric disorders | 32 (16) | 9 (10) | 7 (17) | 10 (26) | 4 (29) | 1 (6) | .03 |
| Treatment before admission | |||||||
| Antiplatelet therapy | 23 (11) | 3 (3) | 8 (19) | 6 (15) | 4 (29) | 2 (12) | .03 |
| ACE inhibitor/ARB | 54 (26) | 8 (9) | 14 (33) | 21 (54) | 7 (50) | 3 (18) | <.01 |
| Statin | 37 (18) | 5 (5) | 12 (29) | 10 (26) | 5 (36) | 5 (29) | <.01 |
| Anticoagulation | 8 (4) | 1 (1) | 2 (5) | 3 (8) | 0 | 2 (12) | .320 |
| Clinical characteristics | |||||||
| Previous infection and/or fever | 68 (33) | 61 (66) | 2 (5) | 3 (8) | 1 (7) | 1 (6) | <.001 |
| Previous stressing event | 20 (10) | 2 (2) | 6 (14) | 10 (26) | 1 (7) | 1 (6) | .004 |
| Heart failure at admission | 14 (7) | 2 (2) | 3 (7) | 6 (15) | 3 (21) | 0 | .032 |
| ECG findings | |||||||
| ST-segment elevation | 118 (57) | 61 (66) | 19 (45) | 27 (69) | 2 (14) | 7 (41) | <.01 |
| Normal ECG | 27 (13) | 11 (12) | 9 (21) | 2 (5) | 1 (7) | 4 (25) | |
| Laboratory values | |||||||
| Troponin ratio | 61 (11-189) | 108 (29-337) | 31 (9-130) | 43 (7-112) | 7 (1-50) | 26 (3-150) | <.001 |
| CKmb, U/L | 22 (10-47) | 36.2(17.5-62) | 17 (8-42) | 15 (9-25) | 11 (4-33) | 9.5 (2-34) | <.001 |
| Hemoglobin, g/dL | 13.5 [12-15] | 14 [13-15] | 13.5 [12-14] | 12.4 [12-14] | 14.4 [12-15] | 13.7 [13-15] | .091 |
| Creatinine, mg/dL | 0.82 [0.7-1] | 0.83 [0.71-0.93] | 0.83 [0.7-1] | 0.72 [0.6-0.95] | 0.91 [0.8-1.04] | 0.84 [0.8-1.05] | <.001 |
| eFGR <60 mL/min/1.73 m2 | 20 (10) | 3 (3) | 5 (12) | 8 (20) | 2 (14) | 2 (12) | .108 |
| Total colesterol, mg/dL | 171 [149-205] | 161 [135-193] | 184 [156-220] | 187 [162-216] | 170 [153-221] | 174 [146-187] | <.001 |
| LDL, mg/dL | 101 [84-131] | 99 [82-129] | 103 [84-140] | 111 [91-131] | 111 [87-145] | 95 [80-109] | .370 |
| HDL, mg/dL | 44 [34-56] | 39 [30-49] | 45 [38-56] | 56 [47-65] | 42 [34-50] | 45 [36-57] | .578 |
| CRP, mg/L | 11.3 [3.6-54] | 31 [10-82] | 5.4 [2-15] | 7.4 [2.4-16] | 6.5 [3.6-61.7] | 5.8 [4-12.5] | <.001 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CAD, coronary artery disease; CK-MB, creatinine kinase myocardial band; CRP, C reactive protein; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HDL: high-density lipoprotein; LDL: low-density lipoprotein; MI: myocardial infarction.
The data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
CMR data according to the diagnostic groups are presented in table 2. Mean left ventricular ejection fraction as well as LV volumes were within the normal values for the overall population. However, when comparing the diagnostic groups, LV ejection fraction was significantly lower and the indexed LV end-diastolic volume significantly larger in the cardiomyopathy group compared with the other groups. Overall, 126 patients (62%) had LV regional wall motion abnormalities. The prevalence of myocardial edema in the whole cohort was 75% based on T2-STIR images, with focal myocardial edema absent in patients with a normal CMR (as per definition). Patients with tako-tsubo cardiomyopathy showed the largest number of LV segments with edema. LGE was observed in 149 patients (72%) and the myocarditis group showed the highest frequency of LGE (94%) followed by patients in the cardiomyopathy group (77%) and in the MI group (74%).
Cardiovascular magnetic resonance characteristics in the overall population and according to diagnostic groups
| Total(N=207) | Myocarditis(n=93) | MI(n=42) | Tako-tsubo(n=39) | Cardiomyopathies(n=14) | Normal(n=17) | P | |
|---|---|---|---|---|---|---|---|
| LVEF, % | 58±10 | 58±8 | 58±10 | 59±10 | 50±18 | 62±6 | .007 |
| iLVEDV, mL/m2 | 77±20 | 79±17 | 77±26 | 69±18 | 91±25 | 73±13 | .015 |
| iLVESV, mL/m2 | 33±17 | 34±11 | 34±24 | 29±14 | 49±28 | 28±7 | .003 |
| iLV mass, g/m2 | 66±19 | 66±19 | 63±20 | 66±14 | 84±26 | 58±12 | .004 |
| RVEF, % | 62±9 | 60±8 | 62±9 | 66±8 | 61±14 | 65±8 | .01 |
| iRVEDV, mL/m2 | 68±17 | 73±15 | 68±17 | 55±16 | 66±16 | 67±12 | <.01 |
| iRVESV, mL/m2 | 26±11 | 29±10 | 26±11 | 19±7 | 27±14 | 24±7 | <.01 |
| Edema | 155 (75) | 82 (88) | 36 (86) | 33 (85) | 2 (14) | 0 | <.01 |
| Segments with edema | 3.4±3 | 3.8±3 | 2.6±2 | 6±3.5 | 0 | 0 | <.01 |
| LGE | 149 (72) | 87 (94) | 31 (74) | 11 (28) | 11 (79) | 4 (25) | <.01 |
iLV, indexed left ventricular; iLVEDV, indexed left ventricular end-dyastolic volume; iLVESV, indexed left ventricular end-systolic volume; iRVEDV, indexed right ventricular end-diastolic volume; iRVESV, indexed right ventricular end-systolic volume; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MI: myocardial infarction; RVEF, right ventricular ejection fraction.
The data are expressed as No. (%) or mean±standard deviation.
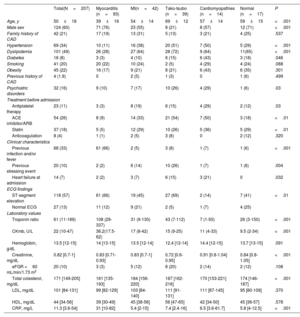
Based on a median time from diagnosis to CMR of 7 days, the cohort was divided in 2 groups: early CMR ( ≤ 7 days, 117 patients) and late CMR group ( >7 days, 88 patients) (figure 3).
Age was the only baseline characteristic that showed significant differences between the early vs late CMR groups (47±18 years vs 54±19 years; P=.01). When we compared CMR findings between the 2 groups, the presence of edema in T2-STIR images was more frequent in the patients who underwent early CMR than in those who underwent late CMR (81% vs 67%; P=.03). In contrast, there were no differences in LV regional wall motion abnormalities and the presence of LGE between early and late CMR. LV ejection fraction was slightly lower in the group of patients who underwent early CMR than in those undergoing late CMR (57% vs 60%; P=.03) (table 3).
Clinical characteristics and CMR findings according to time to CMR
| Early group(CMR ≤ 7 days) (n=117) | Late group(CRM >7days) (n=88) | P | |
|---|---|---|---|
| Time to CMR | 5 d [IQR: 4-6] | 10 d [IQR: 8-12] | |
| Age, y | 47±18 | 54±19 | .01 |
| Male sex | 75 (64) | 47 (53) | .123 |
| Family history of CAD | 20 (17) | 22 (25) | .161 |
| Hypertension | 27 (23) | 31 (35) | .056 |
| Dyslipidemia | 40 (34) | 36 (41) | .324 |
| Diabetes | 8 (7) | 8 (9) | .552 |
| Smoking | 27 (23) | 13 (15) | .138 |
| Obesity | 24 (20) | 21 (24) | .566 |
| Previous history of CAD | 2 (2) | 2 (3) | .782 |
| Psychiatric disorders | 13 (11) | 19 (21) | .045 |
| Treatment before admission | |||
| Antiplatelet therapy | 6 (5) | 17 (19) | .002 |
| ACE inhibitor /ARB | 28 (24) | 26 (29) | .393 |
| Statin | 18 (15) | 19 (21) | .269 |
| Anticoagulation | 2 (2) | 6 (7) | .064 |
| Clinical characteristics | |||
| Previous infection and/or fever | 47 (40) | 21 (24) | .012 |
| Previous stressing event | 10 (9) | 10 (11) | .518 |
| Heart failure at admission | 6 (5) | 8 (9) | .275 |
| ECG findings | |||
| ST-segment elevation | 74 (63) | 44 (50) | .056 |
| Normal ECG | 11 (9) | 16 (18) | .067 |
| Laboratory values | |||
| Troponin ratio | 225 (166-284) | 93 (45-141) | .001 |
| CK-MB, U/L | 36 (29-43) | 38 (25-51) | .797 |
| Hemoglobin, g/dL | 13.7 (13-14) | 13 (12.7-13.4) | .05 |
| Creatinine, mg/dL | 0.82 (0.79-0.86) | 0.87 (0.79-0.95) | .284 |
| eFGR <60 mL/min/1.73 m2 | 8 (7) | 12 (13) | .111 |
| Total colesterol, mg/dL | 178 (169-187) | 194 (159-230) | .319 |
| LDL, mg/dL | 109 (102-116) | 109 (101-118) | .946 |
| HDL, mg/dL | 44 (41-47) | 46 (44-49) | .256 |
| CRP, mg/L | 42 (31-52) | 29 (18-38) | .065 |
| CMR findings | |||
| LVEF, % | 57±9 | 60±10 | .03 |
| iLVEDV, mL/m2 | 76±22 | 77±18 | .763 |
| iLVESV, mL/m2 | 34±18 | 32±15 | .438 |
| iLV mass, g/m2 | 69±20 | 63±17 | .012 |
| RVEF, % | 62±8 | 62±9 | .807 |
| iRVEDV, mL/m2 | 66±17 | 68±17 | .562 |
| iRVESV, mL/m2 | 26±11 | 26±11 | .755 |
| Edema, % | 81 | 67 | .032 |
| Segments with edema | 4±3 | 3±3 | .061 |
| LGE, % | 74 | 66 | .152 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CAD, coronary artery disease; CK-MB, creatinine kinase myocardial band; CMR, cardiovascular magnetic resonance; CRP, C reactive protein; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; iLV, indexed left ventricular; iLVEDV, indexed left ventricular end-dyastolic volume; iLVESV, indexed left ventricular end-systolic volume; IQR, interquartile range; iRVEDV, indexed right ventricular end-diastolic volume; iRVESV, indexed right ventricular end-systolic volume; LDL, low-density lipoprotein; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; RVEF, right ventricular ejection fraction.
Unless otherwise indicated, the data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
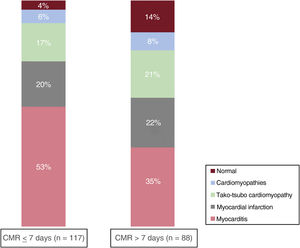
The diagnostic yield of CMR was higher when CMR was performed within the first week (early group) (96% vs 86%; P=.02). Even though myocarditis remained the most common diagnosis in both the early and late CMR groups, this diagnosis was more frequently achieved in the early CMR group (53% vs 35%; P=.01). No differences were observed between the groups of MI or tako-tsubo cardiomyopathy. On the other hand, a normal CMR was more frequently observed in the late CMR group (14% vs 4%; P=.02) (figure 4).
DISCUSSIONThe main findings of the present study were: a) the high etiologic diagnostic yield of comprehensive CMR, allowing a definite diagnosis in 91% of patients with an initial diagnosis of MINOCA, and b) this diagnostic yield further increased to 96% when the CMR was performed within the first week of presentation.
In patients with an initial working diagnosis of MINOCA, it is important to establish an etiological diagnosis to personalize the treatment and provide accurate risk stratification. In recent international practice guidelines, CMR is recommended in patients with an initial diagnosis of MINOCA to define the etiologic diagnosis.1 This recommendation is based on robust evidence that shows that CMR provides a definitive etiologic diagnosis in 64% to 89% of patients with MINOCA.3–9,23,24 In the present study, the use of CMR provided the definite etiologic diagnosis in 9 of 10 patients with an initial diagnosis of MINOCA. The differences in the CMR diagnostic yield observed between the present study and previous literature may be explained by many factors such as the time from initial symptoms to CMR performance. Previous studies with a lower CMR diagnostic yield were those with longer delay between symptom presentation to CMR imaging. In 388 patients diagnosed with MINOCA who underwent CMR within a median time of 37 days (10% of CMR studies performed within 14 days of symptom onset), Dastidar et al.5 showed a diagnostic yield for CMR of 75%. In another study including 719 patients undergoing CMR within 30 days of symptoms, Williams et al.8 reported that the final diagnosis could be achieved in 74% of the patients. In the present study, the CMR was performed within a median time of 7 days, which could explain the higher diagnostic yield compared with previous studies.5,8
After an acute cardiac injury, dynamic changes occur at the myocardial level.25 Such changes include the existence of a bimodal edema pattern during the first week after MI and the fact that the observed edema in CMR starts to disappear after the first week.26 Therefore, if CMR is performed later, there are a significant number of etiologies that can be missed such as small myocarditis or tako-tsubo cardiomyopathy. Sörensson et al.7 compared 2 groups of patients included in Stockholm Myocardial Infarction with Normal Coronaries (SMINC) studies. Although the patients included in the initial SMINC study had comparable clinical characteristics to those included in the SMINC-2 study, in the initial SMINC study, the CMR was performed after a median delay of 12 days and only 20% of the patients showed edema, whereas in the SMINC-2 study, the CMR was performed after a median time delay of 3 days and myocardial edema was detected in more than 75% of patients. These findings were also associated with an improvement in the diagnostic CMR yield in the SMINC-2 study compared with SMINC (77% vs 47%, respectively). As noted by the authors, the higher proportion of patients with edema despite lower mean troponin levels in the SMINC-2 study compared with those of the initial SMINC study could be associated with the higher diagnostic yield of the CMR.7 In the present study, a significantly higher percentage of edema was detected in the early CMR group (81% vs 67%), which could explain the higher CMR diagnostic yield for this group. Specific clinical presentations may result in suspected diagnoses that can lead treating physicians to refer patients to CMR earlier. In the present population, it is noteworthy that 40% of patients undergoing early CMR presented with fever, which suggests the diagnosis of myocarditis.
The final etiological diagnosis distribution after CMR may be partly explained by the timing to CMR, as described previously, but also by the baseline characteristics of the population. Compared with the SMINC-2 study,7 the present population was younger (50 years vs 58 years) and the proportion of male sex was higher (60% vs 29%). These differences could be related to the higher proportion of myocarditis (45% vs 17%) and the lower proportion of tako-tsubo cardiomyopathy (19% vs 35%) described in the present study compared with those reported in the SMINC-2 study.7 In a recent study that included 170 women with an initial diagnosis of MINOCA, myocarditis was the final diagnosis in only 14.7% of patients, pointing to a lower prevalence of myocarditis in women.27
The prevalence of MI as a final diagnosis has consistently been described in previous studies, ranging from 22% to 26%.5–8 More recently, a meta-analysis by Mileva et al.,28 including 26 studies and 3624 patients, showed a prevalence of MI in 22% of the patients. This proportion is similar to that observed in the present study, in which the frequency was 20%.
Study limitationsSeveral limitations should be acknowledged in the present study. This is a single center study with a relatively small number of patients in each diagnostic group, which may underpower the conclusions. Prospective inclusion, but retrospective analysis of patients, may introduce referral bias due to physician decision and logistic considerations for CMR referrals. In 57 (27%) patients, the CMR was performed before coronary angiography, leading to a diagnosis of acute myocarditis in 54 patients. Accordingly, a coronary angiogram was not performed. The remaining 3 patients (1 patient with MI, 1 with tako-tsubo cardiomyopathy, and 1 with cardiomyopathy) had nonobstructive lesions on the coronary angiogram performed after the CMR. Due to the long inclusion period, newer CMR techniques that may increase the diagnostic yield, such as T1 and T2 mapping,18 were not available in all patients.
CONCLUSIONSIn a large consecutive cohort of patients, CMR established the underlying diagnosis in 91% of patients with an initial working diagnosis of MINOCA. The performance of CMR within the first week of presentation further increased its diagnostic yield to 96%. Myocarditis remained the most frequent diagnosis irrespective of the timing of CMR, even though it was more frequent in earlier studies.
FUNDINGNo organization funded the research for this study.
ETHICAL CONSIDERATIONSThe study was performed in accordance with the Declaration of Helsinki and was reviewed and approved by the Institutional Review Board (ethics code: PI-22-048 and PI-19-257). Due to the retrospective analysis of clinically acquired data, the local ethics committee waived the need for written informed consent from patients. Due to the large inclusion period and the retrospective nature of clinical data used, possible sex and gender biases have not been taking into account.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSAll authors have substantially contributed to the conception or design of the work, the acquisition, analysis, or interpretation of the data for the work, drafted the work, or critically revised it for important intellectual content. All authors have granted final approval of the version to be published and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICTS OF INTERESTV. Delgado received speaker fees from Edwards Lifesciences, GE Healthcare, Medtronic, Philips and Novartis and consulting fees from Edwards Lifesciences and Novo Nordisk. A. Bayés-Genís has participated in advisory work and/or lectured for Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Roche Diagnostics and Vifor. The remaining authors have nothing to disclose.
CMR plays an important role in the evaluation of patients with MI with nonobstructive coronary artery disease (MINOCA). However, the timing of the performance of CMR may impact its diagnosis yield. The latest ESC guidelines encourage the performance of CMR within 2 weeks of symptom onset.
WHAT DOES THIS STUDY ADD?The performance of CMR within 7 days of symptom onset allows a higher diagnostic yield in patients with an initial working diagnosis of MINOCA. The findings from this study support advocating for early CMR analysis in patients with MINOCA.