Acute coronary syndromes have a wide spectrum of clinical presentations and risk of adverse outcomes. A distinction should be made between treatable (extent of ischemia, severity of coronary disease and acute hemodynamic deterioration) and untreatable risk (advanced age, prior myocardial damage, chronic kidney dysfunction, other comorbidities). Most of the patients with “untreatable” risk have been excluded from the “guideline-generating” clinical trials. In recent years, despite the paucity of specific randomized trials, major advances have been completed in the management of elderly patients and patients with comorbidities: from therapeutic nihilism to careful titration of antithrombotic agents, a shift toward the radial approach to percutaneous coronary interventions, and also to less-invasive cardiac surgery. Further advances should be expected from the development of drug regimens suitable for use in the elderly and in patients with renal dysfunction, from a systematic multidisciplinary approach to the management of patents with diabetes mellitus and anemia, and from the courage to undertake randomized trials involving these high-risk populations.
Keywords
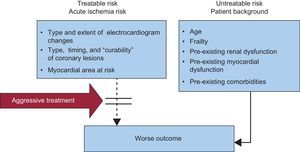
Acute coronary syndromes (ACS) have a wide spectrum of clinical presentations and risk of adverse outcomes. As a general rule in medicine, the higher the baseline risk, the higher the benefit of an early aggressive approach. However, in ACS a distinction should be made between the risk attributable to the acute ischemic event, which may be treatable by an appropriate intervention; and the risk of the patient's background characteristics, comorbidities, and frailty, which may not be treatable and may render the patient more susceptible to the iatrogenic arm (Figure 1).
Risk stratification in acute coronary syndrome. A distinction should be made between treatable and untreatable risk. Aggressive pharmacoinvasive intervention may reduce the prognostic impact of the acute ischemic burden, whereas untreatable-risk variables increase the risk of iatrogenic damage.
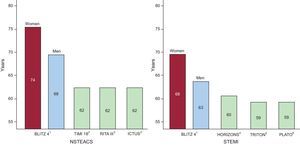
The identification of high-risk patients is easy, and a number of risk stratification tools have been developed, based upon data coming from tens of thousands of patients enrolled in randomized clinical trials (RCT) and registries. On the other hand, deciding how to treat high-risk patients and how to minimize the risk of drug therapy and coronary intervention requires sound clinical judgment, the adoption of specific measures and, in most cases, a multidisciplinary approach. This exercise is often not supported by clinical practice guidelines, as these are mostly based upon the evidence generated from RCTs, which often exclude patients with significant comorbidities and tend to underrepresent elderly patients1–7(Figure 2).
A comparison of mean population ages in the contemporary BLITZ 4 registry of acute coronary syndrome in the Italian coronary care units and the guideline-generating trials for ST-segment elevation myocardial infarction and non—ST-segment elevation acute coronary syndrome. Randomized trials have enrolled patient populations much younger than those observed in clinical practice. NSTEACS, non–ST-segment elevation acute coronary syndrome; STEMI, ST-segment elevation myocardial infarction. Reproduced with permission from Savonitto.7
In the present paper, we will review the current evidence regarding the treatment of ACS in elderly patients and in those with diabetes mellitus (DM), chronic kidney dysfunction (CKD), and hematological abnormalities, which may confuse an early invasive approach to ACS.
THE ELDERLYElderly patients represent a large proportion of patients admitted to hospital with an ACS, and this will be even more the case in the forthcoming decades.8 There is no uniformly accepted definition of “elderly” in terms of age cutoff. The only 2 specific and prospective studies addressing treatment strategies in elderly patients with ACS used the cutoff of ≥ 75 years.9,10 This cutoff is the most commonly used in current literature,11 whereas older papers used lower cutoffs, such as 60 years to 65 years. This issue is important when interpreting the literature, since the relation between age and outcome (particularly mortality, but also bleeding) shows a dramatic worsening around the age of 75.11 Moreover, increasing age does not imply only more years, but also a change in the overall characteristics of the ACS population. The proportion of women increases from<30% when a study population has a mean age of 60 years to 63 years (as in most RCTs) to 50% in study populations with a mean age of 80 years.9,10 Elderly populations will also have>70% hypertensive patients, 35% diabetics, 20% with an estimated glomerular filtration rate (eGFR)<60mL/min, as well as more patients with prior myocardial infarction (MI), stroke, or with atrial fibrillation and peripheral arterial disease.2,3,9–13 All these conditions imply specific problems when deciding treatment strategies. Finally, most RCTs and guidelines on ACS do not consider frailty, which has been shown to profoundly affect outcome in the elderly,14 among the variables to be collected and analyzed to guide treatment strategies.
In 2007, two scientific statements of the American Heart Association Council on Clinical Cardiology reviewed the existing literature on the treatment of non–ST-segment elevation ACS (NSTEACS)15 and ST-segment myocardial infarction (STEMI)16 in the elderly: these documents highlighted the fact that the elderly is largely underrepresented in the study populations of RCTs that form the basis of clinical practice guidelines.
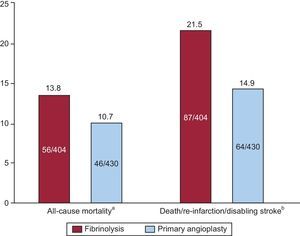
Treatment of ST-segment Elevation Myocardial InfarctionPrimary angioplasty (primary percutaneous coronary intervention [pPCI]) has emerged as the most effective and safe reperfusion strategy in elderly patients with STEMI. The TRIANA was started in 2005 to compare the efficacy and safety of pPCI and fibrinolysis in very old STEMI patients.9 This study, planned to enroll 570 patients, was interrupted after the enrollment of 266 patients over 33 months and showed a trend towards a reduction in the primary endpoint of 30-day death, re-MI, or disabling stroke with pPCI (18.9% vs 25.4%; odds ratio [OR]=0.69; 95% confidence interval [95%CI], 0.38–1.23). The incidence of each of the components of the primary endpoint was directionally lower with pPCI. A pooled analysis of this and two previous RCTs comparing pPCI with lytic therapy, published in the same paper,9 showed a trend towards mortality reduction and a significant reduction in death, re-MI, and stroke at 30 days (Figure 3). The relevance of this RCT data for clinical practice finds confirmation from the analysis of the prospective, multicenter registry of the Reseau de Cardiologie de Franche Comte, comparing 2 periods of time in 2001 and 2006.17 From 2001 to 2006, pPCI became the preferential modality of reperfusion therapy over fibrinolysis (adjusted OR=6.9, 95%CI, 3.1-15), and this change in strategy was associated with a significantly lower 30-day mortality in 2006 (9.2% vs 23.3%; P<.001).
Summary of the meta-analysis of randomized trials comparing fibrinolytic therapy and primary angioplasty in patients with ST-segment elevation myocardial infarction aged ≥ 75 years. aOdds ratio = 0.74 (95% confidence interval, 0.49-1.13; P = .16). bOdds ratio = 0.64 (95% confidence interval, 0.45-0.91; P = .13). Data from Bueno et al.9
A recent meta-analysis including 6298 patients who underwent pPCI and stent implantation included in the Drug-Eluting Stent in Primary Angioplasty (DESERT) Cooperation database12 confirmed that, despite the expected higher rate of death at long-term follow-up in elderly as compared to younger patients (hazard ratio [HR]=2.17; 95%CI, 1.97-2.39; P<0.0001), no impact of age was observed on the risk of re-MI, stent thrombosis, and target vessel revascularization.
A favorable outcome was described with regard to 199 patients aged ≥ 80 years treated within the Minneapolis regional STEMI system:18 the median length of hospital stay was 4 days, in-hospital mortality was 11.6%, and 1-year mortality 25.6%. Of the 166 patients with age ≥ 80 who lived independently or in assisted living before hospital admission and survived, 150 (90.4%) were discharged to a similar living situation or projected to such a living situation after temporary nursing home care.
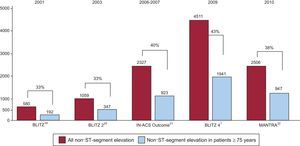
Treatment of Non–ST-segment Elevation Acute Coronary SyndromePatients with non–ST-segment elevation acute coronary syndrome are older than those with STEMI and include more women.1 As shown in Figure 4, in recent Italian ACS registries, patients aged ≥75 years represent approximately 40% of those with NSTEACS.1,19–22 Over the last few years, inferential analyses from registries23 and subgroup analyses of RCTs2,3 have suggested that an early invasive approach is associated with better outcome in elderly patients as compared to conservative treatment. In the multicenter ROSAI-2 registry,23 De Servi et al compared the 30-day outcomes of 564 patients ≥ 75 years of age with those of 1017 younger patients: older patients had worse baseline characteristics and received fewer evidence-based pharmacological therapies. An early aggressive strategy was adopted in 39% of elderly patients and 56% of younger patients (P<.001). An interventional procedure was performed within 30 days in 30% and 48%, respectively, of cases (P<.001). At 30 days, elderly patients had higher rates of death (6.4% vs 1.7%), MI (7.1% vs 5%), and stroke (1.3% vs 0.5%). Multivariate analysis of the elderly group identified a conservative strategy (OR=2.31; 95%CI, 1.20-4.48) and a diagnosis of non–Q-wave MI (OR=2.27; 95%CI, 1.32-3.93) as independent predictors of 30-day events. On the other hand, the aforementioned “Reseau Franche Compte” registry didn’t observe any improvement in 30-day outcome from 2001 to 2006 in elderly patients with NSTEACS despite a four-fold increase in invasive strategy.17 However, it should be considered that a 30-day endpoint may not reflect the advantage of an invasive approach to NSTEACS, which may become evident at a much later time point: longer follow-up has been pursued in RCTs.
Among patients aged ≥ 75 years enrolled in the TACTICS–TIMI 18 trial,2 the early invasive strategy conferred an absolute reduction of 10.8 percentage points (10.8% vs 21.6%; P=.016) and a relative reduction of 56% in death or MI at 6 months, a much higher benefit as compared to that observed in younger age groups. In this study, TIMI (Thrombolysis In Myocardial Infarction) major bleeding rates were almost prohibitive with the early aggressive strategy in patients ≥ 75 years of age (16.6% vs 6.5%; P=.009), most likely due to the systematic upstream therapy with tirofiban and unfractionated heparin, and the universal use of the femoral approach to catheterization.
A collaborative analysis of individual data from the FRISC II, ICTUS and RITA-3 (FIR) trials,3 all comparing routine vs selective invasive strategy in NSTEACS, assessed outcomes up to 5 years after index admission. The composite of cardiovascular death or MI was significantly lower with the routine invasive strategy in patients aged 65 years to 74 years (HR=0.72; 95%CI, 0.58-0.90) and in those aged ≥ 75 years (HR=0.71; 95%CI, 0.55-0.91), but not in those aged<65 years (HR=1.11; 95%CI, 0.90-1.38; P<.001 for interaction between treatment strategy and age). The interaction was driven by an excess of early MI in patients<65 years of age, whereas there was no heterogeneity between age groups concerning cardiovascular death. The benefits were smaller for women than for men (P<0.009 for interaction).
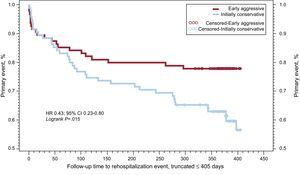
The Italian elderly ACS study10 was the first RCT to enroll exclusively patients with NSTEACS and an age ≥ 75 years: patients were randomized to an early invasive (coronary angiography and, when indicated, revascularization within 72h) or initially conservative (angiography and revascularization only for recurrent ischemia). The study was initially planned to enroll 504 patients with a primary endpoint set at 6-month follow-up, but was subsequently modified to a 12-month primary endpoint with the sample size set at 313 patients; a further 332 patients excluded from the trial for any reason were enrolled in a parallel registry. The mean age of the study population was 82 years, and 50% were women. The primary endpoint (a net clinical benefit of all-cause mortality, MI, disabling stroke, and repeat hospital stay for cardiovascular causes or severe bleeding) was significantly reduced by an early invasive approach in patients with elevated troponin levels on admission (61% of the cases) (Figure 5), though the benefit was not significant in the whole study population (27.9% vs 34.6%; HR=0.80; 95%CI, 0.53-1.19), with a significant treatment per troponin status interaction (P=0.03). The entire component of the primary endpoint trended toward benefit with the early invasive strategy. It is remarkable that, even in such an older population, 80% of the causes of death within 1 year were cardiovascular, and mostly of ischemic origin,24 pointing towards an aggressive treatment both in the acute phase and in secondary prevention. In this contemporary study only 1 adjudicated case (0.6%) of major bleeding was recorded during index admission and 3 subsequent hospital admissions were due to severe bleeding (1.9%).10 This remarkable safety may be due to a high rate (75%) of radial approach to percutaneous coronary intervention (PCI) and a limited (20% in patients undergoing PCI) use of GPIIb/IIIa inhibitors.
Kaplan-Meier survival curves to primary endpoint (all-cause mortality, re-infarction, disabling stroke, and rehospitalization for cardiovascular causes or bleeding) in patients with non–ST-segment elevation myocardial infarction (elevated troponin levels on admission) randomized to an early or to a selective invasive strategy. Data from Savonitto et al.10 Reproduced with permission from Savonitto.7.
Finally in a recent meta-regression analysis including all of the RCTs comparing treatment strategies in NSTEACS, a routine early invasive strategy has been confirmed to reduce the composite endpoint of death and MI (P for interaction=.044), as well as repeat hospitalization (P for interaction<.0001), to a greater extent in elderly than in younger individuals, without significant differences between men and women.25
Cardiogenic Shock and Out-of-hospital Cardiac ArrestElderly patients with cardiogenic shock and those resuscitated from an out-of-hospital cardiac arrest have traditionally been considered off-limits as candidates to PCI in the ACS scenario. A subgroup analysis of the SHOCK trial showed a nonsignificant trend towards increased 30-day mortality (75% vs 53%) when a total of 56 elderly patients with acute MI and cardiogenic shock had received emergency revascularization compared with initial medical stabilization.26 A recent meta-analysis of nonrandomized studies27 has considered a total of 1935 patients with MI and cardiogenic shock aged ≥ 75 years, of which 468 had been treated by emergency revascularization and 1467 with initial medical stabilization. Despite a lower rate of successful revascularization in elderly patients compared with their younger counterparts (n=7 studies; 88% vs 95%; P<.0001), patients who received emergency revascularization experienced lower short-term (55% vs 72%; OR=0.48; 95%CI, 0.33–0.69) and intermediate-term (60% vs 80%; OR=0.47; 95%CI, 0.27–0.83) mortality. However, this kind of analysis does not allow to conclude whether the better outcome is attributable to patient selection rather than to the positive effect of emergency revascularization.
Advanced age was not among the predictors of in-hospital mortality among patients undergoing emergency pPCI after an out-of-hospital cardiac arrest in the registry of pPCI of the Lombardy region in Italy28: in that study, significant independent predictors where the time delay between cardiac arrest and call to the emergency medical system, cardiogenic shock on admission, asystole as initial rhythm and Glasgow coma scale 3. A recent analysis of 6972 patients aged ≥ 65 years (mean age 75.8 [7.0] years), survivors after an out-of-hospital cardiac arrest and discharged alive from hospital,29 a quarter of whom had an acute MI, showed that long-term survival was strongly dependent upon the neurological deficit at discharge and, overall, was not different from mortality after an episode of heart failure. Putting all this information together, it seems reasonable to proceed to emergency angiography in view of possible PCI in elderly patients resuscitated from out-of-hospital cardiac arrest unless the patient had been reanimated from asystole and does not present cardiogenic shock or Glasgow coma scale 3 on admission.
Antithrombotic treatmentAntithrombotic therapy is the mainstay of ACS management, both in patients managed invasively and in those treated conservatively. The use of anticoagulant and antiplatelet agents in elderly patients requires careful tailoring of dosing and prudent evaluation of the bleeding risk. A number of pathophysiological and pharmacological variables affect drug metabolism and disposition in a different way in the elderly as compared to younger patients,30 and a detailed discussion of these specificities goes beyond the scope of the present paper. However, at least the following notes should be retained:
- •
The risk of gastroenteric bleeding with even low-dose acetylsalicylic acid increases with age and previous history of peptic ulcer;31 therefore, administration of a proton pump inhibitor is recommended in these patients.32
- •
In elderly patients who are candidates to fibrinolytic therapy, the use of half-dose weight-adjusted tenecteplase is recommended,33 combined with enoxaparin at the dosage of 0.75mg/kg without the initial intravenous bolus,34 and followed by clopidogrel (75 mg) with no loading dose.
- •
In patients undergoing pPCI, it is reasonable to use bivalirudin rather than unfractionated heparin and a gycoprotein-IIb/IIIa blocker, due to the much lower risk of bleeding.4
- •
In the acute phase of NSTEACS, fondaparinux (2.5 mg once daily) should be the anticoagulant of choice in patients treated conservatively,35 whereas enoxaparin should be dosed very carefully based on the eGFR. The intraprocedural use of bivalirudin has been associated with reduced bleeding in the elderly and also with lower 1-year mortality.36
- •
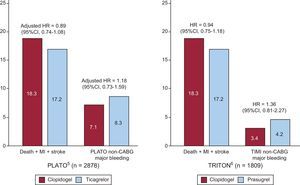
Clopidogrel remains the adenosine diphosphate receptor blocker of choice in most elderly patients. As compared to clopidogrel, the 2 more powerful inhibitors, prasugrel and ticagrelor, had remarkably similar results, with a slight reduction in the ischemic endpoint at the expense of an increased risk of major bleeding (Figure 6).5,6 Therefore, when an adenosine diphosphate receptor blocker is indicated in adjunct to acetylsalicylic acid, the use of these newer agents should be restricted to patients with allergy to clopidogrel, or after careful individual evaluation of the benefit vs risk ratio. The Elderly ACS-2 trial (NCT01777503) is specifically comparing reduced-dose prasugrel (5mg once a day) with clopidogrel in 2000 ACS patients aged ≥ 75 years undergoing early PCI.
Figure 6.Rates of cardiovascular death, nonfatal myocardial re-infarction, nonfatal stroke, and major bleeding within the follow-up period in patients aged ≥ 75 years enrolled in the PLATO5 and TRITON-TIMI 386 trials. Note that: a) the mean length of follow-up was 9.2 months in the PLATO trial, compared to 14.5 months in TRITON-TIMI 38, and; b) the definitions of bleeding were different. Comparison between treatment risk rate is expressed as hazard ratio in TRITON and adjusted hazard ratio in PLATO. 95%CI, 95% confidence interval; CABG, coronary artery bypass graft; HR, hazard ratio; MI, myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction. Reproduced with permission from Savonitto.7
(0.15MB).
According to the Euro Heart survey, 21% of patients with STEMI and 27% of those with NSTEACS had established or newly discovered DM on admission.37 Compared to patients without DM, those with DM have worse in-hospital and long-term outcome38 that is not fully explained by the comorbidities associated with DM, that remains a powerful predictor of worse outcome after adjustment for the most relevant confounders. An exception to this rule may be the elderly, where the excess mortality observed in diabetic patients is mostly attributable to pre-existing CKD and myocardial damage.39 Patients with DM have not been included in the recent decline in mortality observed in patients without DM.40 This might reflect a higher risk factor burden, but also less aggressive treatment.41 Data drawn from registries have consistently shown that patients with ACS and DM are suboptimally treated compared with nondiabetic patients both in terms of revascularizations and with regard to antiplatelet regimen.42,43 This latter issue may be of special relevance, in the face of specific aberrations in platelet function leading to increased on-treatment platelet reactivity.43
An issue partly related to glycemic metabolism in ACS is the prognostic role of hyperglycemia, the prevalence of which ranges from 25% to 50% of patients admitted with ACS.44 Admission blood glucose maintained a powerful and independent effect on mortality up to and beyond 1 year post-MI.45 The strength of association between hyperglycemia on admission and mortality risk after acute MI holds true irrespective of diabetic status46 and may decrease the effect of DM on mortality when adjustment for admission blood glucose is considered.42 Hyperglycemia on admission may be both a marker of an acute stress response (eg, in patients with hemodynamic instability) and an indicator of underlying insulin resistance.44 A Swedish prospective study and a few other reports have suggested that the majority of these patients may in fact have undiagnosed DM.47–49 This may explain why the measurement of fasting glucose level within 24 h of hospitalization and other glucometrics over longer time have improved the ability to predict 30-day mortality.50 Therefore, current guidelines suggest that glucose level should be part of the initial laboratory evaluation in all patients with suspected or confirmed ACS and monitored frequently in patients with known DM or admission hyperglycemia.51,52 Furthermore, the abovementioned guidelines include the following set of recommendations:
- •
Both excessive hyperglycemia (>180–200mg/dL) and hypoglycemia (< 90mg/dL) should be avoided. Despite a growing body of knowledge about the discrimination ability of elevated glucose in predicting mortality in ACS patients and some evidence of improved outcomes from tight glucose control in other critically ill populations,53 studies performed in medical and surgical intensive care units have tempered initial enthusiasm for strict glycemic control.54–56 Indeed, the risk associated with admission glucose level has an U-shaped curve, with an increased rate of events related to both hyperglycemia and hypoglycemia.57
- •
An early invasive strategy is recommended in diabetic patients with NSTEACS. The optimal approach to coronary revascularization should be based on a multidisciplinary evaluation (heart team), and the complexity of coronary disease rather than DM status should be considered in the decision of coronary artery bypass graft surgery vs PCI. Indeed, data drawn from registries and RCTs suggest that coronary artery bypass graft surgery allows better long-term survival in diabetic patients with multivessel disease.58
- •
If a PCI-based strategy is deemed feasible and appropriate, drug-eluting stents should be preferred over bare metal stents. Large prospective registries have confirmed a beneficial effect of drug-eluting stents in DM with a 3% absolute 3-year risk adjusted reduction in all-cause mortality and MI compared to bare metal stents.59 Head-to-head comparison between different drug-eluting stents produced no conclusive evidence about the superiority of one type over another.60
- •
Antithrombotic treatment is indicated as in nondiabetic patients, bearing in mind the abovementioned platelet hyperreactivity in diabetic patients.43 Acetylsalicylic acid resistance has been described in DM and hyperglycemia and the increased platelet turnover rate described in DM has been associated with an increase in thromboxane A2 (TXA2) synthesis.60 Preliminary data suggest that a twice-daily acetylsalicylic acid regimen may improve platelet inhibition in DM, but these data deserve further evaluation in clinical studies.60 Attempts to improve outcome by increasing daily dose of clopidogrel have not been successful.61 Rather, the third-generation adenosine diphosphate receptor blockers, prasugrel and ticagrelor, provide more potent and predictable platelet inhibitory effects. In a predefined subgroup analysis of the DM cohort, the reduction in the primary ischemic endpoint was consistent with the overall results with ticagrelor62 and even more profound with prasugrel,63 without increased bleeding as compared to clopidogrel.
- •
Patients with ACS and hyperglycemia but without prior history of DM should have further evaluation to determine the severity of their metabolic derangements. This evaluation may include fasting glucose and glycohemoglobin assessment and a postdischarge oral glucose tolerance test.52
Glomerular filtration rate (GFR) progressively declines with age at an approximate rate of 8mL/min/1.73 m2 per decade, and a large proportion of elderly people have a GFR < 60mL/min/1.73 m2. Beginning at the age of 30 years, a process of glomerular replacement by fibrous tissue, called glomerulosclerosis, takes place, affecting an increasing number of glomeruli with increasing age.64 Aging is also accompanied by a decrease in creatinine production (senile sarcopenia), and therefore serum creatinine levels do not increase proportionally with the progressive decrease in GFR. Therefore, a serum creatinine of 1mg/dL corresponds to a GFR of 120mL/min at the age of 20, but only 60mL/min in an 80-year-old person. Because of the complexity of direct measurement of GFR by infusion of exogenous substances, formulas have been proposed to estimate GFR from serum concentrations of endogenous markers that are filtered by the glomeruli. Creatinine-based equations, such as the Modification of Diet in Renal Disease and the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula contain age and sex as variables, whereas only the Cockcroft-Gault formula also takes into account body weight. All these equations underestimate the directly measured GFR by 20% to 25%, the most likely reason being that these equations were developed in patients with decreased muscle mass, compared with healthy adults.65 As a result, older patients with an eGFR of 45mL to 59mL/min/1.73 m2 are less likely than younger patients to progress to end-stage renal disease, and when progression occurs it is slower than that in younger patients.66 Moreover, elderly patients have a lower age-adjusted risk of dying than younger patients with similar eGFR.
Although CKD is a well-known risk variable in NSTEACS,67 its role in dictating prognosis in elderly patients with NSTEACS has not been determined so far. In the Italian Elderly ACS study, about 60% of the patients had an eGFR<60mL/min/1.73 m2 when the CKD-EPI or the Modification of Diet in Renal Disease formula were used, as compared to 80% when the Cockcroft-Gault equation was applied.68 Analyzed as a continuous variable, eGFR was among the independent predictors of mortality,24 and in this respect the Cockroft-Gault formula fared significantly better than the other two equations.68
The clinical manifestations of ACS are often atypical in patients with CKD, and the diagnostic challenge may be increased by the fact that serum concentrations of troponins are frequently raised in elderly patients with CKD.
Patients with CKD are more prone to bleeding complications than those with preserved renal function. Defects in platelet adhesion and aggregation leading to hemorrhagic tendency have been described in patients with CKD.69 Therefore, they should be cautiously treated with antithrombotic agents exclusively or substantially eliminated through the kidney, such as the small-molecule glycoprotein-IIb/IIIa inhibitors and enoxaparin, drugs that need to be down-titrated according to the eGFR. Dosing errors in CKD patients, mostly at prescription, account for a substantial part of adverse drug reactions in ACS patients. In severe CKD, enoxaparin and fondaparinux are contraindicated: in such patients unfractionated heparin should be used, having also the advantage that its anticoagulant activity is easily monitored using the activated partial thromboplastin time, and it can be quickly neutralized in the event of bleeding. The dose of bivalirudin also must be reduced in patients with severe CKD.
Among the P2Y12 receptor antagonists, ticagrelor showed strikingly favorable results as compared with clopidogrel in the PLATO trial, significantly reducing ischemic endpoints and mortality in patients with CKD.70 Although major and fatal bleedings were not significantly increased by ticagrelor, they were numerically higher in patients allocated to ticagrelor than in those assigned clopidogrel, particularly in patients with severe CKD (23.6% vs 14.1%).
The great majority of elderly patients with CKD are not treated invasively because many physicians are reluctant to use coronary angiography in this setting, due to the risk of further deterioration in GFR. This underuse leads to underdiagnosis of significant coronary artery disease and to lower rates of subsequent revascularization. Moreover, the potential beneficial effects of an invasive strategy cannot be adequately assessed, as only a few patients with severe CKD have been included in RCTs comparing invasive and conservative strategies. The few available data come from published registries: among these, the SWEDEHEART registry reported data on 5689 patients with non—ST-segment elevation myocardial infarction and ≥ stage 3 CKD.71 Those with lower eGFR were less likely to undergo coronary angiography and revascularization within 14 days of admission. In patients who underwent coronary angiography, declining renal function was associated with more severe coronary artery disease. The adjusted 1-year mortality was 36% lower with an invasive strategy, yet the difference in mortality observed with invasive therapy declined with lower renal function, with no difference in mortality in patients with an eGFR of<15mL/min/1.73 m2. It must be noted, however, that patients>80 years old were excluded from the analysis. The “therapeutic nihilism” adopted in CKD patients also extends to measures of secondary prevention, such as advice for smoking cessation, weight loss, exercise, and cardiac rehabilitation. Moreover, drugs like statins, β blockers, and antiplatelet agents are less frequently prescribed at discharge in patients with than without CKD.72
ANEMIAAnemia, defined according to the World Health Organization criteria (hemoglobin<13 g/dL in men or<12 g/dL in women),73 is found in 15% to 20% of ACS patients.74 The 2011 European Society of Cardiology guidelines for the management of NSTEACS identify anemia as an important risk factor for both ischemic and bleeding complications.51 The 2012 update of European Society of Cardiology STEMI guidelines mentions anemia as a risk factor for worse outcomes, suggesting that dual antiplatelet therapy should be undertaken with caution.75 They suggest to accurately measure hemoglobin for risk stratification and pay attention to antithrombotic treatment, type of stent, and vascular access (preferring the radial approach); they also suggest a restrictive transfusion threshold, reserving blood compounds to unstable hemodynamic status or hematocrit<25% or hemoglobin level<7g/dL. However, they note that the management of this patient group is based on empirical data.
Evidence drawn from the recent literature adds the following findings and suggestions:
- •
Systematic review of adjusted analyses suggests an increased risk for all-cause mortality in anemic patients, [1-year adjusted HR 1.63, 95%CI 1.10-2.40], with a dose-dependent effect.76
- •
Anemia may affect outcome by the combination of decreased oxygen delivery to myocardium downstream of coronary stenoses and increased myocardial oxygen demand, and it is the first cause of type II MI, defined by an imbalance in oxygen delivery.77
- •
Anemia, either at baseline or acquired during hospitalization, provides independent prognostic information for bleeding risk and for prediction of mortality or recurrent MI on top of traditional ischemic prognostic tools. Adding anemia on admission to traditional scores for ischemic risk may allow almost 50% of ACS patients to be reclassified into a lower risk category.78
- •
Anemia is quite common in elderly patients with acute cardiovascular disease, being found in up to 43% of this patient group.79 In this setting, anemia is associated with a higher prevalence of comorbidities, including type 2 DM, CKD, chronic heart failure, and cerebrovascular disease. However, its independent prognostic role holds true even after adjustment for age, sex, and the most clinically relevant covariates and frailty status.24,80
- •
Interventions aimed at correcting anemia, such as packed red blood cell transfusion, may be associated with worse outcomes. Indeed, in the setting of MI, liberal blood transfusions have been associated with a weighted absolute risk increase of 12% and a number needed to harm of 8 (95%CI, 6-17).81 However, most of the literature focusing on transfusion strategies includes small, pilot RCTs or observational studies and may be affected by a confounding higher risk profile of anemic patients.82
- •
Intravenous iron carboxymaltose helps to alleviate symptoms in patients with heart failure and iron deficiency, improving exercise tolerance and quality of life and decreasing cardiovascular events. However, the long-term health implications are uncertain and further studies are warranted.83
- •
Erythropoiesis-stimulating agents do not seem to guarantee beneficial effects on mortality, cardiovascular events, and hospitalizations in patients with mild to moderate anemia and heart disease and may be associated with serious harms. A large RCT assigned 2278 patients with systolic heart failure and hemoglobin levels of 9 g/dL to 12g/dL to darbepoietin titrated to a target hemoglobin level of 13g/dL or placebo: there were no differences in health outcomes and thromboembolic events in the intervention group after a median 28-month follow-up.84 However, no trials in patients with heart disease have evaluated the effects of more moderate hemoglobin level targets (10-12g/dL) compared with lower targets.
Thus, patients with ACS and anemia have an increased prevalence of multiple comorbidities, a high-risk cardiovascular profile, and an overall worse outcome. The complex medical condition of this cohort imposes a great challenge for therapeutic decision-making; specific guidelines with recommended medical treatment and invasive strategies tailored to the various clinical conditions are warranted. Clinicians should minimize the bleeding risk using available risk models, and should adjust the type and dose of antithrombotic agents. Radial access for cardiac catheterization and PCI should be used whenever possible. Every effort should be made, beginning in the acute phase, to identify the cause of anemia because the finding of gastrointestinal blood loss has important implications for subsequent antithrombotic management.
CONCLUSIONSIn recent years, despite the paucity of specific RCTs trials, major advances have been made in the management of elderly patients and patients with comorbidities: from therapeutic nihilism to careful titration of antithrombotic agents, a shift toward the radial approach to percutaneous coronary interventions, and also to less-invasive cardiac surgery. Further advances should be expected from the development of drug regimens suitable for use in the elderly and in patients with renal dysfunction, from a systematic multidisciplinary approach to the management of patents with DM and anemia, and from the courage to undertake randomized trials involving these high-risk populations.
CONFLICTS OF INTERESTNone declared.
Section sponsored by AstraZeneca