Hypertension is highly common in heart failure (HF). However, there is limited information on its prevalence, circadian variation, and relationship with the various HF phenotypes. The objective of this study was to describe the prevalence of hypertension and its patterns in HF.
MethodsThis was a cross-sectional observational study of patients with optimized stable chronic HF. The patients underwent blood pressure (BP) measurement in the office and 24-hour ambulatory monitoring. We estimated the prevalence of hypertension, and its diurnal (controlled, uncontrolled, white coat, and masked) and nocturnal (dipper, nondipper, and reverse dipper) patterns. We also analyzed the factors associated with the different patterns and HF phenotypes.
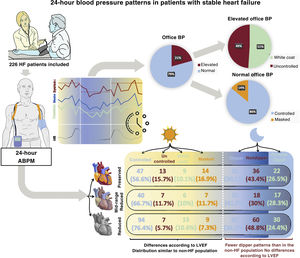
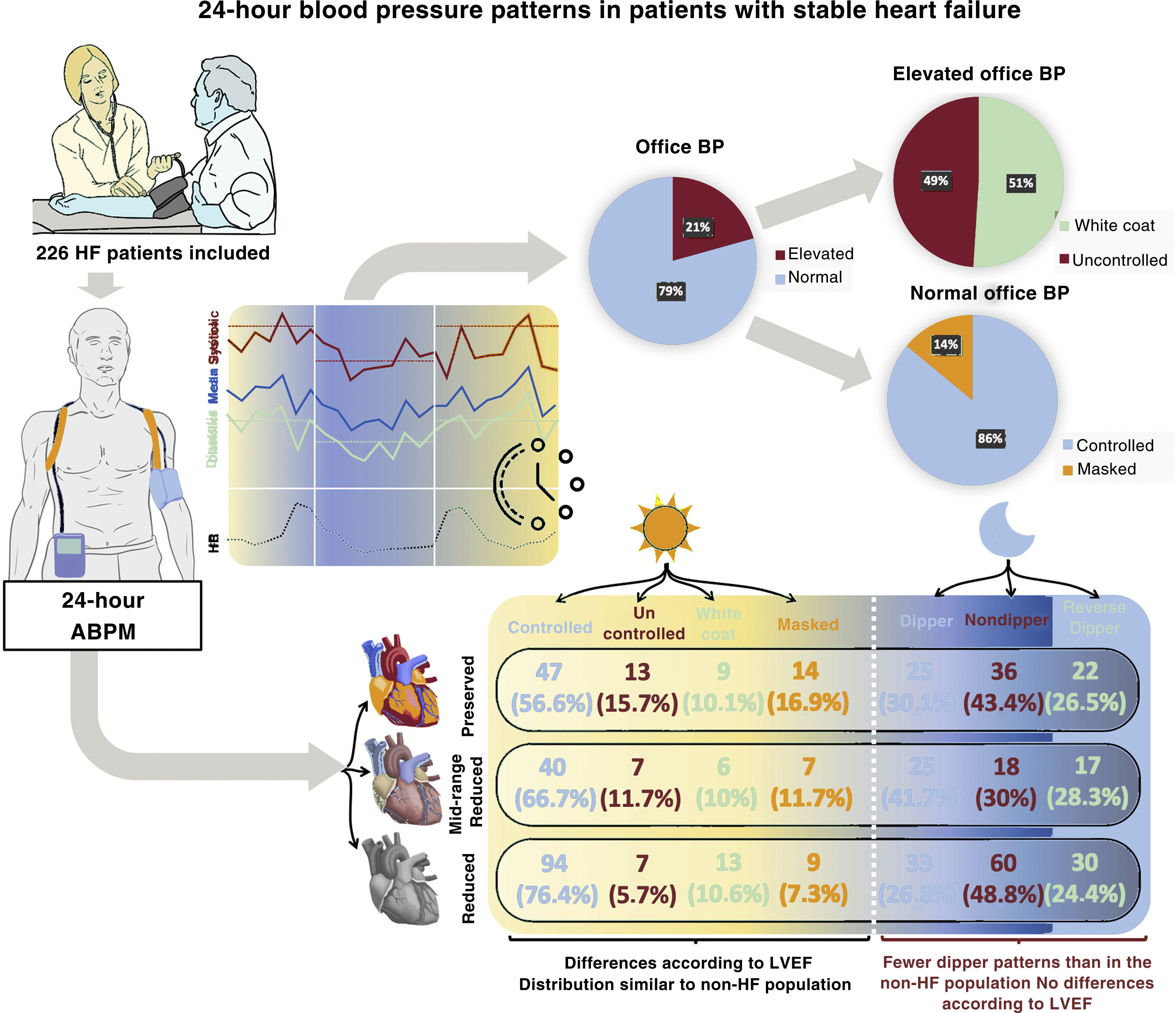
ResultsFrom 2017 to 2021, 266 patients were included in the study (mean age, 72±12 years, 67% male, 46% with reduced ejection fraction). Hypertension was present in 83%: controlled in 68%, uncontrolled in 10%, white coat in 10%, and masked in 11%. Among patients with high office BP, 51% had white coat hypertension. Among those with normal office BP, 14% had masked hypertension. The prevalence of dipper, nondipper, and reverse dipper patterns was 31%, 43%, and 26%, respectively. Systolic BP was lower in HF with reduced ejection fraction than in HF with preserved ejection fraction (P <.001).
ConclusionsAmbulatory BP monitoring in HF identified white coat hypertension in more than half of patients with high office BP and masked hypertension in a relevant percentage of patients. The distribution of daytime patterns was similar to that of the population without HF in the literature, but most of the study patients had a pathological nocturnal pattern.
Keywords
Hypertension (HT) is the most significant risk factor for the development of cardiovascular disease,1,2 and is an established risk factor for heart failure (HF), in which it plays a decisive role in the onset of the clinical syndrome, contributing to both systolic and diastolic dysfunction (50% of HF cases are attributed to HT). 3
The estimated prevalence of HT in HF patients is around 90%.3 The risk of HF progressively increases as systolic and diastolic blood pressure (BP) values rise above 120 and 80mmHg, respectively.4,5
The prognostic role of single brachial BP measurements in the physician's office is limited in hypertensive patients.6 Ambulatory blood pressure monitoring (ABPM) provides values that are more in line with reality and better related to the presence of target organ damage,7 cardiovascular events, and mortality.8–11 Furthermore, ABPM yields information on the features of nocturnal BP, enabling identification of specific patterns, such as white coat and masked HT, the latter having recognized prognostic value in hypertensive patients.10,12
HF generates various hormonal changes, which, together with the recommended treatment for this disease, lead to changes in both the BP and heart rate.13,14 Little information is available on office BP values, their relationship with ABPM values, or the nocturnal dynamics of this parameter in HF. Thus, the aim of this descriptive study was to determine the prevalence and factors associated with various diurnal HT patterns (controlled, uncontrolled, masked, and white coat) and nocturnal patterns (dipper, nondipper, and reverse dipper) in a large population of HF patients. In addition, we investigated associations between these patterns and the patients’ HF phenotypes, defined according to left ventricular ejection fraction (LVEF).
METHODSObservational, cross-sectional study with prospective data collection, carried out between 2017 and 2021 in 2 Spanish teaching hospitals with HF units. The study was approved by the Drug Research Ethics Committee (CEIm, Comité de Ética de la Investigación con Medicamentos) of Hospital Universitario 12 de Octubre, with identification number 17/225. Informed consent was obtained and filed from all patients participating in the study.
Study populationThe study included consecutive patients with stable HF (more than 1 month without decompensation, clinical worsening, or treatment changes), who were enrolled if their physician considered their treatment optimized according to definitions in clinical practice guidelines.14 Based on the LVEF values, estimated using the biplane Simpson method, patients were classified as follows: HF with reduced ejection fraction (HFrEF) when LVEF was ≤ 40%, mid-range (HFmrEF) when 41% to 49%, and preserved (HFpEF) when ≥ 50%. The most recent LVEF value at the time of the study, always within the previous year, was taken as the reference. We excluded patients younger than 18 years, those with changes in their clinical status in the previous month, those requiring hypotensive medication adjustments in the medical visit, and those refusing to participate or sign the informed consent form.
Drugs used for BP control without an HF indication were considered other antihypertensives or additional antihypertensives.
Blood pressure measurementOffice BP measurement was performed with a validated semiautomated oscillometric device (OMRON M6 Comfort), with the patient in sitting position and after a 5-minute rest. The value used in the analysis was the mean of 2 determinations obtained 2 minutes apart. The measurements mainly used a standard-diameter cuff (25-35cm for office and 24-32cm for ambulatory testing), although occasionally a larger or smaller cuff was required, according to patient size. When there was a significant difference (>10mmHg) between the 2 determinations, a third was obtained and the discordant determination was eliminated. Within the first month after enrollment, patients underwent 24-hour ABPM with an automatic oscillometric device validated for this purpose (Mobil-O-Graph and Spacelabs). The cuff was placed on the nondominant arm and BP measurements were taken every 15 to 20 min during the day and every 20 to 30minutes at night. Diurnal and nocturnal periods were delineated based on information provided by the patient. Recordings with at least 70% of valid measurements were included in the analysis.
Definitions of blood pressure patternsBased on the ABPM results, the following variables were obtained: mean 24-hour systolic BP (SBP), mean 24-hour diastolic BP (DBP), mean diurnal SBP, mean diurnal DBP, mean nocturnal SBP, and mean nocturnal DBP.
Patients were classified into the following groups based on office BP values and ABPM results:
- •
Controlled HT (or no HT if there was no previous diagnosis): office SBP <140mmHg and DBP <90mmHg, with mean 24-hour SBP <130mmHg and mean 24-hour DBP <80 mmHg on ABPM.
- •
Uncontrolled HT (or new HT diagnosis): office SBP ≥ 140mmHg and/or DBP ≥ 90mmHg, with mean 24-hour SBP ≥ 130mmHg and/or mean 24-hour DBP ≥ 80mmHg on ABPM.
- •
White coat HT (white coat effect in patients previously diagnosed with HT): office SBP ≥ 140mmHg and/or DBP ≥ 90mmHg, with mean 24-hour SBP <130mmHg and mean 24-hour DBP <80mmHg on ABPM.
- •
Masked HT (or uncontrolled masked HT in patients previously diagnosed with HT): office SBP <140mmHg and DBP <90mmHg, with mean 24-hour SBP ≥ 130mmHg and/or mean 24-hour DBP ≥ 80mmHg on ABPM.
Nocturnal ABPM patterns:
- •
Dipper: mean SBP decrease ≥ 10% at night compared with the daytime value.
- •
Extreme dipper: mean SBP decrease ≥ 20% at night compared with the daytime value.
- •
Nondipper: mean SBP decrease 0% to 10% at night compared with the daytime value.
- •
Riser (or reverse dipper): mean SBP increase at night compared with the daytime value.
Most of the population included (79%) had been diagnosed with HT before the study. To facilitate the analysis and data presentation, we decided to group patients showing analogous BP patterns on ABPM: no HT together with controlled HT, newly diagnosed HT with uncontrolled HT, white coat HT with white coat effect, and masked HT with uncontrolled masked HT. We will refer to each group by the HT nomenclature. Similarly, as there were very few patients with the extreme dipper nocturnal pattern, they were placed in the dipper pattern group.
Calculation of the total HT prevalence in our population included all forms of HT. The only patients excluded from the numerator were those with no history of HT and office and ABPM values within the normal range.
Statistical analysisQuantitative variables are expressed as the mean±standard deviation or the median [interquartile range], and qualitative variables as the absolute and relative frequency. The chi-square or Fisher exact test was used to compare qualitative variables. ANOVA or the Kruskal-Wallis test and the Student t or Mann-Whitney U test, when appropriate, were used to compare quantitative variables. Baseline characteristics were compared between the various HT patterns. The normality of the distribution was evaluated using the Kolmogorov-Smirnov test.
Multivariate logistic regression analysis was performed to assess the factors associated with the HT patterns of interest (white coat, masked, and reverse dipper HT); results are reported as odds ratios (OR) and 95% confidence intervals (95%CI). The initial model included variables with a P value <.1 on univariate analysis. Variables from the initial model were eliminated using a backward stepwise procedure with the cutoff at P <.05. Data analyses were done with STATA, version 15.0 (Stata Corp., United States).
RESULTSTotal populationWithin the period covering August 2017 to February 2021, 360 patients were recruited for the study and 266 were analyzed (). Baseline characteristics are shown in table 1. Mean age was 71.8±12 years, 177 (66.5%) were men, and 210 (79%) had a previous HT diagnosis. Ten additional hypertensive patients (3.8%) were diagnosed during the study, giving a total HT prevalence of 82.7%. Among the 266 patients included, 123 (46.2%) had HFrEF, 60 (22.6%) HFmrEF, and 83 (31.2%) HFpEF.
Baseline characteristics in relation to blood pressure phenotypes
| Factor, n | Total (N=266) | Controlled HT (n=181) | Uncontrolled HT(n=27) | P | White coat HT(n=28) | P | MaskedHT (n=30) | P | P Groups |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 71.8±12.0 | 71.1±11.8 | 71.0±11.2 | .95 | 75.9±11.6 | .048 | 72.5±13.9 | .55 | .26 |
| Male sex | 177 (66.5) | 130 (71.8) | 13 (48.1) | .013 | 13 (46.4) | .007 | 21 (70.0) | .84 | .01 |
| BMI | 27.8±4.6 | 27.6±4.5 | 28.0±5.1 | .68 | 28.4±4.6 | .34 | 28.3±5.0 | .42 | .72 |
| HT | 210 (79.0) | 135 (74.6) | 26 (96.3) | .012 | 21 (75.0) | .96 | 28 (93.3) | .023 | .01 |
| DM | 97 (36.5) | 56 (30.9) | 14 (51.9) | .032 | 11 (39.3) | .38 | 16 (53.3) | .017 | .03 |
| DL | 157 (59.0) | 100 (55.2) | 19 (70.4) | .14 | 15 (53.6) | .87 | 23 (76.7) | .028 | .08 |
| Smoking | .32 | ||||||||
| Nonsmoker | 130 (48.9) | 84 (46.4) | 14 (51.9) | .74 | 19 (67.9) | .10 | 13 (43.3) | .49 | |
| Smoker | 21 (7.9) | 16 (8.8) | 3 (11.1) | 1 (3.6) | 1 (3.3) | ||||
| Exsmoker | 115 (43.2) | 81 (44.8) | 10 (37.0) | 8 (28.6) | 16 (53.3) | ||||
| AF | 137 (51.5) | 101 (55.8) | 8 (29.6) | .011 | 14 (50.0) | .57 | 14 (46.7) | .35 | .08 |
| COPD | 34 (12.8) | 19 (10.5) | 3 (11.1) | .92 | 4 (14.3) | .55 | 8 (26.7) | .014 | .10 |
| SAHS | 42 (15.8) | 26 (14.4) | 4 (14.8) | .95 | 5 (17.9) | .63 | 7 (23.3) | .21 | .64 |
| AS | 24 (9.02) | 18 (9.9) | 1 (3.7) | .29 | 1 (3.6) | .27 | 4 (13.3) | .57 | .42 |
| PAD | 28 (10.5) | 15 (8.3) | 5 (18.5) | .093 | 2 (7.1) | .84 | 6 (20.0) | .047 | .11 |
| NYHA | |||||||||
| Class I | 59 (22.2) | 39 (21.5) | 3 (11.1) | .44 | 7 (25.0) | .27 | 10 (33.3) | .33 | .34 |
| Class II | 180 (67.7) | 126 (69.6) | 21 (77.8) | 16 (57.1) | 17 (56.7) | ||||
| Class III | 27 (10.2) | 16 (8.8) | 3 (11.1) | 5 (17.9) | 3 (10.0) | ||||
| LVEF | |||||||||
| Preserved | 83 (31.2) | 47 (26.0) | 13 (48.1) | .024 | 9 (32.1) | .78 | 14 (46.7) | .041 | .06 |
| Mid-range | 60 (22.6) | 40 (22.1) | 7 (25.9) | 6 (21.4) | 7 (23.3) | ||||
| Reduced | 123 (46.2) | 94 (51.9) | 7 (25.9) | 13 (46.4) | 9 (30.0) | ||||
| Rhythm | |||||||||
| Sinus | 154 (57.9) | 98 (54.1) | 22 (81.5) | .015 | 20 (71.4) | .23 | 14 (46.7) | .73 | .06 |
| AF/flutter | 74 (27.8) | 54 (29.8) | 5 (18.5) | 5 (17.9) | 10 (33.3) | ||||
| PMK/CRT | 38 (14.3) | 29 (16.0) | 0 | 3 (10.7) | 6 (20.0) | ||||
| Analyses | |||||||||
| Hemoglobin, g/dL | 13.7±1.8 | 13.9±1.8 | 13.0±1.3 | .014 | 13.7±1.6 | .69 | 13.8±1.9 | .87 | .10 |
| Creatinine, mg/dL | 1.2 [0.97-1.49] | 1.2 [1.0-1.4] | 1.1 [0.8-1.7] | .53 | 1.3 [1.0-1.5] | .59 | 1.2 [0.9-1.8] | .78 | .85 |
| eGFR (CKD-EPI), mL/min | 49 [36-65] | 48 [38-64] | 50 [32-88] | .34 | 49 [40-63] | .98 | 52.5 [31-78] | .82 | .80 |
| Sodium, mEq/L | 141±3 | 141.4±2.8 | 142.7±3.1 | .020 | 141.6±3.1 | .63 | 141.1±2.3 | .57 | .09 |
| Potassium, mEq/L | 4.6±0.5 | 4.6±0.5 | 4.7±0.6 | .42 | 4.8±0.5 | .046 | 4.5±0.7 | .46 | .18 |
| NT-proBNP, pg/mL | 1.161 [551-2.557] | 1.204 [586-2.844] | 1.168 [405-2.152] | .48 | 841 [536-1.550] | .27 | 1.009 [466-2.114] | .57 | .64 |
| Treatment | |||||||||
| ACEI | 80 (30.1) | 52 (28.7) | 11 (40.7) | .21 | 8 (28.6) | .99 | 9 (30.0) | .89 | .65 |
| ARB | 56 (21.1) | 33 (18.2) | 10 (37.0) | .024 | 5 (17.9) | .96 | 8 (26.7) | .28 | .12 |
| Sacubitril-valsartan | 89 (33.5) | 73 (40.3) | 3 (11.1) | .003 | 9 (32.1) | .41 | 4 (13.3) | .004 | .002 |
| Beta-blocker | 232 (87.2) | 162 (89.5) | 21 (77.8) | .081 | 25 (89.3) | .97 | 24 (80.0) | .14 | .21 |
| MRA | 148 (55.9) | 115 (63.9) | 9 (33.3) | .003 | 8 (28.6) | <.001 | 16 (53.3) | .27 | <.001 |
| Ivabradine | 24 (9.0) | 17 (9.4) | 2 (7.4) | .74 | 3 (10.7) | .82 | 2 (6.7) | .63 | .94 |
| Diuretic | 206 (77.4) | 138 (76.2) | 21 (77.8) | .86 | 21 (75.0) | .89 | 26 (86.7) | .20 | .64 |
| Other antihypertensives | 46 (17.3) | 21 (11.6) | 12 (44.4) | <.001 | 2 (7.1) | .48 | 11 (36.7) | <.001 | <.001 |
ACEI, angiotensin converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; AS, acute stroke; BMI, body mass index; CRT, cardiac resynchronization therapy; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; DL, dyslipidemia; DM, diabetes mellitus; HT, hypertension; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal fraction of probrain natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral arterial disease; PMK, pacemaker; SAHS, sleep apnea-hypopnea syndrome
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
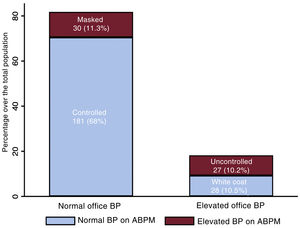
Office BP measurement yielded a median value of 120/70mmHg (105/63-133/80) and a mean of 120/72±20/12mmHg. The distribution of BP values in the population is shown in .
Diurnal blood pressure patternsAmong the 266 patients included, 181 (68%) had controlled HT, 27 (10.2%) uncontrolled HT, 28 (10.5%) white coat HT, and 30 (11.3%) masked HT. Fifty-five patients (20.7%) had elevated office BP values, of which 28 (50.9%) were due to white coat HT. Among the 211 (79.3%) patients with normal office BP values, 30 (14.2%) had masked HT (figure 1). Patients with uncontrolled HT were more often women and this group had a higher prevalence of diabetes mellitus (DM), HT, and HFpEF. Uncontrolled HT was associated with less frequent use of sacubitril-valsartan and aldosterone antagonists, and greater prescription of additional nonspecific antihypertensive agents for HF. Patients with white coat HT were older and more often women. Those with masked HT had a higher comorbidity burden (HT, DM, dyslipidemia, chronic obstructive pulmonary disease, and peripheral arterial disease), a greater prevalence of HFpEF, more frequent prescription of additional antihypertensives, and less sacubitril-valsartan use.
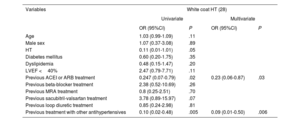
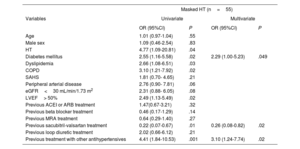
In patients with elevated office BP, logistic regression analysis identified the following independent factors associated with fewer cases of white coat HT: ongoing treatment with angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and other antihypertensive drugs (table 2). Among those with normal office BP values, DM and treatment with other antihypertensives were associated with masked HT, whereas ongoing treatment with sacubitril-valsartan was related to a lower presence of this HT form (table 3).
Factors associated with white coat HT in patients with office HT
| Variables | White coat HT (28) | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| OR (95%CI) | P | OR (95%CI) | P | |
| Age | 1.03 (0.99-1.09) | .11 | ||
| Male sex | 1.07 (0.37-3.08) | .89 | ||
| HT | 0.11 (0.01-1.01) | .05 | ||
| Diabetes mellitus | 0.60 (0.20-1.75) | .35 | ||
| Dyslipidemia | 0.48 (0.15-1.47) | .20 | ||
| LVEF <40% | 2.47 (0.79-7.71) | .11 | ||
| Previous ACEI or ARB treatment | 0.247 (0.07-0.79) | .02 | 0.23 (0.06-0.87) | .03 |
| Previous beta-blocker treatment | 2.38 (0.52-10.69) | .26 | ||
| Previous MRA treatment | 0.8 (0.25-2.51) | .70 | ||
| Previous sacubitril-valsartan treatment | 3.78 (0.89-15.97) | .07 | ||
| Previous loop diuretic treatment | 0.85 (0.24-2.98) | .81 | ||
| Previous treatment with other antihypertensives | 0.10 (0.02-0.48) | .005 | 0.09 (0.01-0.50) | .006 |
95%CI, 95% confidence interval; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; HT, hypertension; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; OR, odds ratio
Factors associated with masked HT in patients with normal office blood pressure values
| Masked HT (n=55) | ||||
|---|---|---|---|---|
| Variables | Univariate | Multivariate | ||
| OR (95%CI) | P | OR (95%CI) | P | |
| Age | 1.01 (0.97-1.04) | .55 | ||
| Male sex | 1.09 (0.46-2.54) | .83 | ||
| HT | 4.77 (1.09-20.81) | .04 | ||
| Diabetes mellitus | 2.55 (1.16-5.58) | .02 | 2.29 (1.00-5.23) | .049 |
| Dyslipidemia | 2.66 (1.08-6.51) | .03 | ||
| COPD | 3.10 (1.21-7.92) | .02 | ||
| SAHS | 1.81 (0.70- 4.65) | .21 | ||
| Peripheral arterial disease | 2.76 (0.90- 7.81) | .06 | ||
| eGFR<30 mL/min/1.73 m2 | 2.31 (0.88- 6.05) | .08 | ||
| LVEF> 50% | 2.49 (1.13-5.49) | .02 | ||
| Previous ACEI or ARB treatment | 1.47(0.67-3.21) | .32 | ||
| Previous beta blocker treatment | 0.46 (0.17-1.29) | .14 | ||
| Previous MRA treatment | 0.64 (0.29-1.40) | .27 | ||
| Previous sacubitril-valsartan treatment | 0.22 (0.07-0.67) | .01 | 0.26 (0.08-0.82) | .02 |
| Previous loop diuretic treatment | 2.02 (0.66-6.12) | .21 | ||
| Previous treatment with other antihypertensives | 4.41 (1.84-10.53) | .001 | 3.10 (1.24-7.74) | .02 |
95%CI, 95% confidence interval; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HT, hypertension; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; OR, odds ratio; SAHS, sleep apnea-hypopnea syndrome.
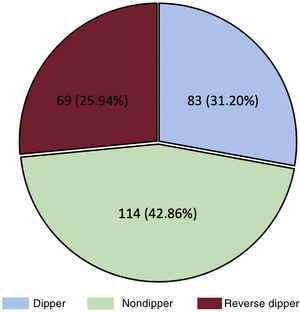
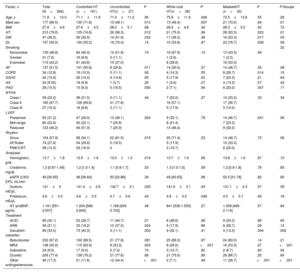
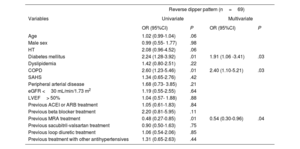
The most frequent nocturnal pattern was the nondipper pattern (114 patients, 42.9%), followed by the dipper pattern (83, 31.2%) and reverse dipper (69, 25.9%) (figure 2). Baseline characteristics of these groups are shown in . The median night/day SBP ratio was 0.9 (0.9-1.0) and the mean night/day was 0.95±0.1. The median night/day DBP ratio was 0.9 (0.8-1.0) and the mean night/day was 0.9±0.1. The nondipper profile was associated with poorer functional class and greater presence of HFrEF. The reverse dipper pattern was observed in older patients, with a greater presence of HT, DM, and ivabradine use. DM and chronic obstructive pulmonary disease were independently associated with a higher risk of the reverse dipper pattern, whereas treatment with aldosterone antagonists showed an inverse association (table 4). There was no association between the office BP and the nocturnal BP pattern.
Factors associated with a reverse dipper pattern
| Reverse dipper pattern (n=69) | ||||
|---|---|---|---|---|
| Variables | Univariate | Multivariate | ||
| OR (95%CI) | P | OR (95%CI) | P | |
| Age | 1.02 (0.99-1.04) | .06 | ||
| Male sex | 0.99 (0.55- 1.77) | .98 | ||
| HT | 2.08 (0.96-4.52) | .06 | ||
| Diabetes mellitus | 2.24 (1.28-3.92) | .01 | 1.91 (1.06 -3.41) | .03 |
| Dyslipidemia | 1.42 (0.80-2.51) | .22 | ||
| COPD | 2.60 (1.23-5.46) | .01 | 2.40 (1.10-5.21) | .03 |
| SAHS | 1.34 (0.65-2.76) | .42 | ||
| Peripheral arterial disease | 1.68 (0.73- 3.85) | .21 | ||
| eGFR <30 mL/min/1.73 m2 | 1.19 (0.55-2.55) | .64 | ||
| LVEF> 50% | 1.04 (0.57- 1.88) | .88 | ||
| Previous ACEI or ARB treatment | 1.05 (0.61-1.83) | .84 | ||
| Previous beta blocker treatment | 2.20 (0.81-5.95) | .11 | ||
| Previous MRA treatment | 0.48 (0.27-0.85) | .01 | 0.54 (0.30-0.96) | .04 |
| Previous sacubitril-valsartan treatment | 0.90 (0.50-1.63) | .75 | ||
| Previous loop diuretic treatment | 1.06 (0.54-2.06) | .85 | ||
| Previous treatment with other antihypertensives | 1.31 (0.65-2.63) | .44 | ||
95%CI, 95% confidence interval; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HT, hypertension; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; OR, odds ratio; SAHS, sleep apnea-hypopnea syndrome
The SBP results were significantly lower in HFrEF than in the remaining HF groups for all the measurements performed (office, 24-hour, nocturnal, and diurnal) ()
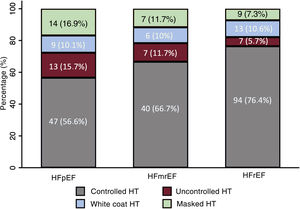
The HT patterns differed between the various HF groups, defined according to LVEF. Patients with HFrEF showed a higher percentage of controlled HT than the HFpEF group (76.4% vs 56.6%; P <.001) and a similar percentage of white coat HT (figure 3). In contrast, these patients had a lower prevalence of uncontrolled (5.7% vs 15.7%; P=.018) and masked (7.3% vs 16.9%; P=.033) HT than patients with HFpEF.
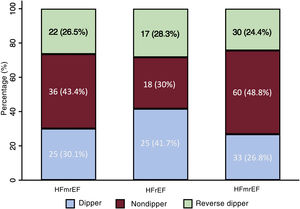
In general, there were no differences in nocturnal patterns between the LVEF groups (P=.17), and the reverse dipper pattern showed a similar prevalence (P=.84). A nonsignificant trend to greater presence of the dipper pattern was observed in HFmrEF (P=.12) (figure 4).
DISCUSSIONThe main value of this study is that it offers a comprehensive view of diurnal and nocturnal BP patterns in a large population of patients with stable chronic HF, receiving optimal medical treatment, and with representation of the entire LVEF spectrum (figure 5). The results show the clinical usefulness of ABPM in chronic HF patients and provide the following relevant findings: a) ABPM enables identification of frequent BP patterns (10.5% prevalence of white coat and 11.3% of masked HT); b) considerable changes occur in the nocturnal BP pattern; and c) there are specific variations in the BP pattern according to LVEF.
Information on BP is scarce in HF patients, and certain treatments affect this parameter; hence, it is of particular interest to study BP features in this population. More than 80% of our patient sample had HT. Most had been diagnosed before the study, but an additional 4% were identified after enrollment. The distribution of diurnal BP patterns in the HF population was similar to that of healthy persons and HT patients, but the nocturnal patterns notably differed. White coat HT, seen in up to half of patients with elevated BP in medical visits, especially in older patients with high cardiovascular risk,15 was found in 50% of our population. Masked HT, observed in 11% of participants, is estimated at 8% to 17% in the general population.16,17 The finding that office BP results differed from those obtained with ABPM (white coat and masked HT) in 22% of patients in our study underlines the importance of ABPM in HF patients, as these patterns would not have been detected otherwise. However, the large volume of HF patients warrants selection of those who should undergo ABPM. For this reason, it may be useful to have identified the independent factors associated with these patterns in our study.
Of particular note, nocturnal BP measurement showed a high prevalence of abnormal patterns in our patients. Only 31% of participants had a dipper pattern, which is seen in around 70% of the healthy population and in HT patients with the same criteria as those in our study.18 Various pathophysiological mechanisms may be involved in BP changes: an increase in sympathetic tone with a decrease in circadian BP variability, a rise in circulating blood volume in recumbent position in a person whose heart cannot effectively handle the preload increase, and the frequent involvement of abnormal renal function.19 The dipper pattern may also be more prevalent in patients with lower concentrations of circulating catecholamines and normal blood volume. There is some evidence that nondipper and reverse dipper patterns have a negative prognostic impact,9,20–24 but the information is scarce in HF patients.25,26 A higher risk of HF-related hospitalization and death has been described in elderly patients with chronic HF and a nondipper pattern. In addition, one study showed a higher prevalence of reverse dipper in HFpEF and another reported that this pattern was associated with greater total and cardiovascular mortality in HF patients.27,28 However, these last 2 studies were performed in patients with acute HF. In contrast, the results of the present study show a similar percentage of nocturnal patterns among the various HF types.
A common feature of most published studies on this subject is that the samples included are not representative of all HF patients. Although 1 recent study found a similar distribution of nocturnal patterns,26 it did not reflect the daytime patterns or provide an analysis stratified by LVEF values, as was done here. An ongoing registry of ABPM results in HFpEF patients will shed light on this population.29 The observation that SBP is lower in HFrEF makes sense from the pathophysiological perspective, as myocardial contractility loss will lead to lower cardiac output, which is related to lower BP.13 This would also explain the higher prevalence of controlled HT and the lower prevalence of masked HT seen in HFrEF. In addition, this population is treated more often with neuromodulatory drugs, which influence the prognosis of the disease and lower BP. Nonetheless, there were no differences in the nocturnal patterns according to the LVEF, a finding possibly related to a persistent increase in sympathetic tone throughout the HF spectrum.13,30
Of note, although the definition of blood pressure patterns is uniform, the diagnostic criteria used in the literature vary considerably. This study used the definitions cited most often, which are those with the greatest scientific support and consensus.8,23,26,31
There is abundant evidence on target values in hypertensive individuals,31–33 but the optimal values in HF patients are unknown.14,31 Although clinical practice guidelines recommend starting medical treatment when BP is > 140/90mmHg, they also indicate that values <120/70mmHg should be avoided because of the higher risk of events in HF patients with low BP. A necessary previous step is to establish BP values and patterns in HF; the present study attempts to contribute information in this line.
Thus, this study supports the value of ABPM for diagnosing prevalent HT patterns (white coat and masked HT) that would not be identified otherwise and indicates that nocturnal BP patterns in HF likely differ from those in other populations, and it describes BP features based on LVEF.
LimitationsDespite the value of describing the prevalence and characteristics of BP patterns in a large HF population, our study has several limitations. This is a cross-sectional, observational study and is therefore subject to the particularities of this type of analysis. The cross-sectional design reflects patient characteristics at a specific moment in time but does not allow evaluation of BP, LVEF, or treatment over the course of their disease. Furthermore, although patients with the entire spectrum of LVEF values were included, they came from the HF units of 2 hospitals with a predominance of patients hospitalized in the previous months and a greater presence of HFrEF. Therefore, these units may not represent all HF patients in our area, and the results may not be applicable to other settings. Finally, the possible prognostic impact of the various BP patterns remains to be described.
CONCLUSIONSThe prevalence of HT is high in HF patients. ABPM was useful for assessing BP profiles in this population: white coat HT was identified in more than half of patients with elevated office BP regardless of their LVEF status, and there was a considerable percentage of masked HT. The distribution of diurnal patterns was similar to that described in populations without HF, but the physiological dipper pattern was found in only one-third of patients studied. In HFrEF patients, SBP was lower and there was a higher presence of controlled HT.
- –
HT is a major risk factor for HF and treatment for HT can prevent HF. Diurnal and nocturnal BP patterns and factors related to their development are well defined in the population without HF. However, the BP features determined by ABPM and their relationship with office BP measurement and the patients’ profile are uncertain.
- –
We describe the prevalence of BP patterns determined by ABPM in an HF population encompassing the entire LVEF spectrum and analyze the patient characteristics and factors related to the development of these patterns. The data are further explained based on the LVEF interval of the sample included. This provides knowledge of the BP features in this disease, influenced by cardiac function and concomitant medication, and enables development of new studies to evaluate their prognostic impact and possible treatment strategies.
None.
AUTHORS’ CONTRIBUTIONSJ. de Juan Bagudá, A. Rodríguez Chaverri, P. Caravaca Pérez, J. de la Cruz, L.M. Ruilope and J.F. Delgado Jiménez contributed to the study design. J. de Juan Bagudá, A. Rodríguez Chaverri, P. Caravaca Pérez, F. Aguilar Rodríguez, M.D. García-Cosío Carmena, S. Mirabet Pérez, M.L. López and J.M. Guerra contributed to data collection. J. de Juan Bagudá, P. Caravaca Pérez, J. de la Cruz and J.F. Delgado Jiménez contributed to the data analysis. All authors took part in writing and revising the manuscript.
CONFLICTS OF INTERESTM.D. García-Cosío Carmena has received fees for scientific presentations from Chiesi and AstraZeneca. L.M. Ruilope has received fees for consultancy and expert testimony from Bayer, and for scientific presentations from Daiichi-Sankyo, Novartis, Medtronic, Pfizer, ReCor, Sandoz, and Sanofi. The remaining authors declare no conflicts of interest.
The authors thank Ana Benito, Mercedes Ferrón, and Alejandro Cruz for their collaboration in data collection for the article.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.02.018