This article discusses the main advances in cardiac arrhythmias and pacing published between 2013 and 2014. Special attention is given to the interventional treatment of atrial fibrillation and ventricular arrhythmias, and on advances in cardiac pacing and implantable cardioverter defibrillators, with particular reference to the elderly patient.
Keywords
The elevated prevalence of atrial fibrillation (AF) in Spain1 and its high social and health impact is driving scientific developments in AF ablation, particularly in the development of different techniques and candidate selection.
Overall Outcomes of Ablation in Different PopulationsThe efficacy of first AF ablation procedures in an optimum candidate (paroxysmal AF without heart disease or atrial dilatation) is between 60% and 80%. Data from a recently published meta-analysis unanimously confirmed that ablation was clearly superior to pharmacological treatment, with a recurrence of 28% and 65%, respectively.2 The CAMTAF3 survey showed that ablation was also superior to medical treatment in patients with heart failure, depressed ejection fraction (EF), and persistent AF. Ablation not only improved EF (from 32 [8%] to 40 [12%]), but also quality of life, oxygen consumption, and B type natriuretic peptide levels. However, this study showed that a single procedure had only moderate efficacy (38%), as 11 of 26 patients (42%) required at least a second procedure.
The RAAFT-2 study showed that ablation was superior to antiarrhythmic drugs as a first treatment for paroxysmal AF, although the recurrence rate was 55% after a suboptimal first procedure.4
This year saw the publication of the results of the Spanish SARA multicenter study, which was the first randomized controlled trial to compare the efficacy of catheter ablation with antiarrhythmic drug treatment in patients with persistent atrial fibrillation.5 In 146 patients, the efficacy of ablation was clearly superior to antiarrhythmic drugs (70.4% and 43.7%, respectively) in suppressing the number of AF episodes per year, with an absolute risk reduction of 26.6%.
The rate of severe procedure-related complications has decreased (especially complications related to pulmonary vein stenosis) and is currently between 1% and 5%.6
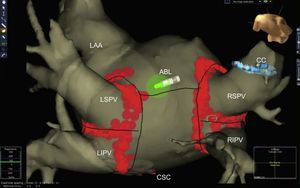
Advances in Techniques and Ablation StrategyPulmonary vein isolation by radiofrequency catheter ablation using nonfluoroscopic navigation systems remains the standard AF ablation technique (Figure 1), regardless of the use of segmented images of the left atrium obtained by computed tomography angiography or magnetic resonance imaging (MRI). New diagnostic maneuvers during AF ablation include documenting local capture and exit block in a pulmonary vein during pacing from the ipsilateral pulmonary vein. This maneuver confirms the electrical isolation of the pulmonary veins, which are connected by endocardial (carina) or epicardial connections in more than 80% of cases.7
Left atrium obtained by multislice computed tomography segmentation and non-fluoroscopic navigation after atrial fibrillation ablation (circumferential pulmonary vein isolation) ABL, ablation catheter; CC circular catheter; CSC, coronary sinus catheter; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Randomized trials have yet to confirm the potential usefulness of catheters that monitor the direction and degree of contact between the tip and the atrium.8
Second-generation balloon cryoablation has an efficacy of around 80%.9 In Spain, 80% of hospitals providing AF ablation perform less than 50 such procedures per year,6 so the availability of a simple technique with a short learning curve is particularly important. These characteristics are associated with cryoablation. A study conducted in a similar hospital described efficacy and safety data that were apparently comparable with those obtained in cryoablation series from 10 high-volume hospitals.10
The TTOP-AF study11 on phased ablation using multielectrode radiofrequency catheters reported a 44% recurrence at 6 months in patients with persistent AF and long-standing persistent AF. Asymptomatic thrombus formation in the ablation catheter and pulmonary vein stenosis raises doubts concerning this technique, in addition to the already known problem of silent cerebral lesions associated with this technique.
Finally, in patients with paroxysmal AF, the identification of stable rotors as ablation guides has recently been shown to have better efficacy at 1 year than pulmonary vein isolation alone (82% and 45%, respectively).12 However, in general, the favorable outcomes of these types of additional ablation strategies have not been replicated in subsequent studies.
Clinical Practice Guidelines UpdatesThe 2104 American Heart Association, American College of Cardiology, and Heart Rhythm Society practice guidelines13 consider symptomatic paroxysmal AF refractory to ≥ 1 antiarrhythmic drug a class I indication (level of evidence A) for ablation and a class IIa indication if AF is persistent. Ablation as first-line therapy is considered acceptable (class IIb), although only in patients with paroxysmal AF and without structural heart disease (Table 1). Other than isolating the pulmonary veins, no specific recommendations are provided on the ablation strategy to be followed in patients with nonparoxysmal AF.
Indications for Atrial Fibrillation Catheter Ablation to Maintain Sinus Rhythm13
| Class I |
| 1. Symptomatic paroxysmal AF refractory or intolerant to at least 1 class I or III antiarrhythmic drug (level of evidence: A) |
| Class IIa |
| 1. Symptomatic persistent AF refractory or intolerant to at least 1 class I or III antiarrhythmic drug (level of evidence: A) |
| 2. Recurrent symptomatic paroxysmal AF before starting antiarrhythmic drugs, after weighing the risks and benefits of drug and ablation treatment (level of evidence: B) |
| Class IIb |
| 1. Persistent long-term symptomatic AF refractory or intolerant to at least one antiarrhythmic drug class I or III (level of evidence: B) |
| 2. Symptomatic persistent AF before initiating antiarrhythmic drugs (level of evidence: C) |
| Class III |
| 1. Patients who cannot be treated with anticoagulants during and after the procedure (level of evidence: C) |
| 2. AF ablation should not be performed with the sole intention of discontinuing anticoagulation (level of evidence: C) |
AF, atrial fibrillation.
Adapted from January et al.13 with permission.
After 16 years of experience in AF ablation, efficacy and procedure-related complications rates are excellent, although there is still room for improvement. In addition to the impact of technical progress and improvements in operator skill, in the near future further improvement may be due to patient selection rather than type of AF, atrial size, and the presence of structural heart disease.
In 2014, 3 new clinical characteristics related to atrial remodeling—apnea-hypopnea, the extent of atrial fibrosis, and atrial sphericity (the latter 2 characteristics quantified by MRI)—have been confirmed as strong predictors of arrhythmia recurrence after ablation (in up to 70% of cases).14–16 Prevalence data (> 50% of patients with AF have these 3 characteristics to some degree) and the rate of arrhythmia recurrence suggest that these characteristics should be screened for prior to ablation.
Percutaneous Closure of the Left Atrial AppendageOne of the most studied topics in 2014 was percutaneous closure of the left atrial appendage as adjunctive treatment of AF (Figure 2). Most studies of percutaneous closure using the Amplatzer device17,18 or Watchman device19 have shown the noninferiority of these devices to warfarin. Procedural success rates have clearly improved (between 95% and 98%) and complications rates have been reduced (between 2% and 4%) in patients with absolute contraindications to anticoagulation therapy17,18 and in patients without them.19 In addition, cost-effectiveness studies have shown the clear benefit of this strategy.20
Finally, the combined strategy of AF ablation and percutaneous closure of the left atrial appendage has emerged as a promising and highly attractive hybrid treatment in subgroups of patients with symptomatic AF who require suspension of anticoagulation therapy.21
Developments in the Pharmacological Treatment of Atrial Fibrillation: Antiarrhythmic Drugs and AnticoagulantsA study on dronedarone that included 4856 patients suggested that, apart from its debated antiarrhythmic efficacy, concerns regarding liver disease and sudden death from proarrhythmia may have been exaggerated, as no deaths from either cause were observed in this large population.22
According to the recently published AFBAR registry,23 42% of patients with AF undergoing heart rate control take digoxin either alone or in combination with beta blockers. Despite previous evidence, digoxin use is not associated with increased all-cause mortality, all-cause hospitalization, or hospitalization in patients with heart failure.
The assessment and comparison of the new oral anticoagulants for stroke prevention remains a current and pressing issue.
This year saw the publication of a study that compared the new drug edoxaban with warfarin. Edoxaban was noninferior to warfarin in the prevention of embolic events and there were fewer major bleeding events (20%) and fewer deaths from cardiovascular causes.24 The results of several registries have also been published on the use of the new oral anticoagulants in the real world and have shown that their efficacy and the risk of bleeding are similar to those observed in clinical trials.25,26 The use of the new oral anticoagulants have results similar to those of warfarin in other clinical situations, such as during cardioversion.27 These data have led to the publication of a specific guideline on this issue.28
Winkle et al29 have suggested replacing anticoagulation therapy with antiplatelet therapy in patients with prior stroke who have undergone effective AF ablation (no recurrence 2 years after ablation). This suggestion should be taken with caution and freedom from AF should be demonstrated, because other studies have found that the withdrawal of anticoagulation therapy is an independent predictor of mortality.
Previous registries have shown that anticoagulant therapy is underused in elderly patients with AF, but none has done so as clearly as the registry of stroke survivors with atrial fibrillation conducted in Glasgow.30 Anticoagulation therapy was prescribed in only 34% in these patients (mean age 78 years) and age was clearly associated with the nonprescription of anticoagulant therapy.
ADVANCES IN THE TREATMENT OF VENTRICULAR ARRHYTHMIASSubstrate Ablation of Ventricular TachycardiaBipolar voltage mapping is useful for identifying high-voltage areas within the scar and is a classic method to identify slow conduction channels, which are critical isthmus sites in ventricular tachycardia circuits and the target of ablation procedures.31 However, the ratio of slow conduction channels identified by this method to the ventricular tachycardia isthmuses has been challenged in a recent study showing that only 30% of slow conduction channels identified by voltage mapping were associated with the isthmus site of any ventricular tachycardia.32 In addition, a recent study in a series of patients with arrhythmogenic right ventricular dysplasia showed that only 40% of the isthmus sites of induced ventricular tachycardia were found in slow conduction channels identified by voltage mapping.33 This study identified more slow conduction channels by tagging the electrograms with delayed components that are sequentially activated than those found by voltage mapping, including most (60%) of the slow conduction channels forming the ventricular tachycardia isthmus, thus suggesting the need to incorporate the former method in the substrate ablation of ventricular tachycardia.
The use of late enhancement cardiac MRI to identify and characterize the arrhythmia substrate has proven to be very useful in planning and guiding ventricular tachycardia ablation procedures. In a recent series, most ventricular tachycardia ablation procedures (96%) were performed in segments with delayed enhancement, whose presence in epicardium identified the epicardial origin of the ventricular tachycardia with a sensitivity of 80% and a specificity of 89%.34 Other recent studies have suggested that ventricular tachycardia ablation procedures could be facilitated by visualizing the 3 -dimensional structure of the substrate by using 3 -dimensional cardiac MRI with integrated electroanatomic mapping systems.35
Visualizing the substrate can be complicated in patients with nonischemic cardiomyopathy, because they commonly have isolated pockets of scar tissue in the midportion of the interventricular septum that is masked by the surrounding healthy tissue during bipolar mapping. A recent study has shown that mapping during right ventricular basal septal pacing in patients with septal scar revealed slowed conduction areas and the presence of late potentials that were not observed during sinus rhythm mapping.36
Recent studies have proposed the use of selective techniques to isolate the substrate or eliminate slow conduction channels. These techniques include circumferential ablation with electrical isolation of the substrate37 or scar dechanneling using multipolar catheters to selectively ablate relatively earlier late potentials in the delayed component.38
Ablation of Premature Ventricular ComplexPremature ventricular complex ablation in patients with depressed EF has recently been shown to be associated with progressive clinical and functional improvement (including improved EF), regardless of the cause of ventricular dysfunction and whether it was induced or worsened by premature ventricular complex. A baseline premature ventricular complex burden > 13% identified patients who would benefit from ablation.39
Implantable Cardioverter Defibrillators: Avoiding Inappropriate Therapies and Stratifying the Risk of Sudden DeathThe recent ADVANCE III randomized trial40 analyzed the effect of long detection settings CE in primary and secondary prevention patients. An increase from the nominal setting of 18 to 24 detection intervals to 30 to 40 detection intervals was associated with a 25% reduction in implantable cardioverter defibrillator (ICD) treatments and a 34% reduction in shocks.
The risk stratification of sudden death after myocardial infarction remains a topic of investigation. Zaman et al41 analyzed the usefulness of electrophysiological study to guide ICD implantation in the acute postinfarction stage in a series of patients undergoing primary angioplasty who presented EF < 40% in the first 4 days after myocardial infarction. Patients with EF < 30% or < 35% and functional class NYHA II/III with a negative electrophysiology study result had ventricular arrhythmia-free survival and a sudden death rate similar to patients with EF > 40%. These findings suggest that a negative electrophysiological study could identify patients with severely depressed EF in the acute postinfarction phase as being at low risk of sudden death and not needing ICD implantation. On the other hand, Izquierdo et al42 analyzed the additional value of measuring infarct size by cardiac MRI for predicting arrhythmic events in patients undergoing primary angioplasty. They found that almost all of the adverse events at 2 year follow-up were observed in patients with large infarcts (> 23.5g/m2) and EF < 35%, whereas none were observed in patients with severely depressed EF and small infarcts during the same period.
These studies demonstrate the low specificity of EF in predicting arrhythmic events and the need to develop new tools to optimize the selection of patients for ICD implantation in the acute postinfarction phase.
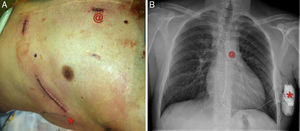
Regarding new technologies, a totally subcutaneous ICD (Figure 3) with exclusively extravascular components is now available for routine use in some patient subgroups.43 Although there is a lack of randomized studies, the results of the prospective EFFORTLESS S-ICD registry44 in a cohort of almost 500 patients suggest that the subcutaneous ICD has efficacy and event rates similar to those of conventional ICDs and prevents the complications, sometimes severe, associated with intracavitary electrodes.
The mean age of patients implanted with an ICD is increasing. The most recent Spanish ICD Registry (2012),45 which included pediatric patients with ICDs, reported a mean age at implantation of 62 years (range, 7-90 years). The Italian ClinicalService Project group46 analyzed more than 6300 patients who underwent ICD implantation and demonstrated the importance of age and comorbidity in the vital prognosis of these patients. These data are of special relevance in elderly patients, because death often has a noncardiac cause in this group. This year, a joint document was published by the Spanish Society of Geriatrics and Gerontology, the Spanish Society of Palliative Care, and the Geriatric Cardiology Section of the Spanish Society of Cardiology47 on the management of the patients with ICDs in the final stages of life. It is increasingly common to find elderly people with ICDs with advanced end-stage disease, including refractory heart failure, oncological diseases, organ failure, or other neurodegenerative diseases with poor short-term prognosis. The article addressed the process of decision making in the final stage of life and presented an algorithm indicating the steps to follow from the time the patient requests an ICD until the time of its deactivation. However, to establish recommendations applicable to clinical practice, consensus documents need to be developed that include the knowledge and perspective of all the subspecialties, particularly electrophysiology and cardiac pacing, involved in this clinical context.
Conte et al48 published a study that described the relatively benign prognosis of Brugada syndrome in a group of elderly patients (N = 437). The best outcomes were observed in the subgroup of older patients (older than 70 years;n = 25). After a mean follow-up of 54 (18) months, no patients older than 70 years experienced arrhythmic events.
NEW DEVELOPMENTS IN CARDIAC PACINGLeadless Pacemakers and Biological SystemsTwo studies have been published that may represent a paradigm shift in the field of medium- to long-term cardiac pacing. Reddy et al49 tested the safety and clinical performance of a leadless pacemaker implanted in 33 patients. The VVIR device, which includes the electronic circuits, lithium battery, and leads, was implanted in the right ventricular apex. The implantation success rate was 97%. One patient had cardiac perforation and tamponade and subsequently died as a result of stroke. The R-wave, impedance, and pacing threshold values at 3 months were stable and similar to those of conventional devices with active fixation leads. Hu et al50 studied the effects of minimally invasive somatic reprogramming in 12 pigs. After complete heart block was induced, the pigs were randomized to receive a percutaneous injection of green fluorescent protein (control group) or the Tbx18 gene into the right ventricle. Gene delivery reprogrammed ordinary cardiomyocytes and converted them into cells with the characteristics of the sinoatrial node.
Sleep Apnea SyndromeA prospective European study51 that included 40 patients compared the apnea-hypopnea index assessed by polysomnography with the respiratory disturbance index assessed by a pacemaker transthoracic impedance sensor featuring the Sleep Apnea Monitoring algorithm. An optimal cutoff value for the respiratory disturbance index of 20 events/h identified patients with pacemakers with severe apnea-hypopnea syndrome with a sensitivity of 89%, a positive predictive value of 89%, and a specificity of 85%.
Minimum Ventricular Pacing SystemsIn a randomized trial, Botto et al52 investigated whether the minimization of ventricular pacing using AAI (R) or DDD (R) mode was superior to standard DDD mode in patients without permanent atrioventricular block. Although a significant reduction was observed in the rate of ventricular pacing (minimal ventricular pacing 5% vs DDD, 86%; P < .0001), there was no significant difference in the primary endpoint (cardiovascular hospitalization) or secondary targets (persistent AF, permanent AF, and composite of death and cardiovascular hospitalization).
Pacing Alternative SitesLau et al53 recently compared whether pacing at the right atrial appendage vs the low right atrial septum can prevent the development of persistent AF in 385 patients with paroxysmal AF and sick sinus syndrome. The study found that pacing at the right atrial septum did not prevent the development of persistent AF at 3.1 years of follow-up. A prospective, randomized, double-blinded, crossover study compared EF after long-term His or para-His pacing with right ventricular septal pacing.54 The primary endpoint was the effect on EF, which was significantly lower after 1 year of right ventricular septal pacing than after 1 year of His pacing. There were no significant differences in clinical parameters, quality-of-life assessments, or complications, but pacing thresholds were significantly more stable and lower in right ventricular septal pacing leads.
Remote Monitoring of Cardiac PacingThe HomeGuide Registry55 and the REFORM56 and TRUST57 clinical trials suggest that remote monitoring is safe, enables longer in-office follow-up intervals, and improves adherence to scheduled follow-up. The recent European Heart Rhythm Association registry58 has described the current state of this technology in Europe.
NEW DEVELOPMENTS IN CARDIAC RESYNCHRONIZATION THERAPYMild Heart Failure, Narrow QRS Complex, or Moderate Ventricular DysfunctionThe results of the REVERSE study59 and the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT)60 suggest that the benefit of cardiac resynchronization therapy in asymptomatic or mild heart failure patients is maintained at a follow-up of 5 years. However, the NARROW-CRT61 and EchoCRT62 studies showed that cardiac resynchronization therapy does not benefit patients with a QRS complex duration ≤ 120 ms-130ms.
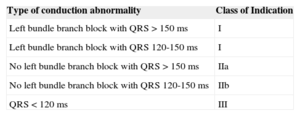
Based on the evidence provided by these and other studies, the latest guidelines on cardiac resynchronization therapy have highlighted the limited benefit of this technique in patients with narrow QRS complexes (Table 2).63 Finally, a substudy of the REVERSE study suggested that cardiac resynchronization therapy produced similar clinical benefit in patients with EF between 30% and 40% and patients with more severe ventricular dysfunction.64
Indications for Cardiac Resynchronization Therapy in Outpatients with Sinus Rhythm, Functional Class II, III, or IV Heart Failure, Receiving Optimal Drug Therapy, and Ejection Fraction ≤ 35%.57
| Type of conduction abnormality | Class of Indication |
|---|---|
| Left bundle branch block with QRS > 150 ms | I |
| Left bundle branch block with QRS 120-150 ms | I |
| No left bundle branch block with QRS > 150 ms | IIa |
| No left bundle branch block with QRS 120-150 ms | IIb |
| QRS < 120 ms | III |
Adapted from Varma et al.57 with permission.
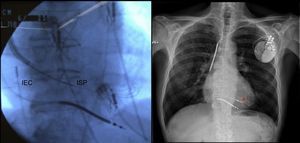
The use of additional selection criteria, such as beta-adrenergic receptors65 or cardiac magnetic resonance assessment of dyssynchrony,66 could lead to improved response to cardiac resynchronization therapy. In addition, some studies published this year suggest that left ventricular pacing sites with greater electrical67 or mechanical68 delay are associated with improved response, whereas multisite left ventricular pacing can significantly improve acute LV hemodynamic parameters.69 The WiSE-CRT70 study investigated the feasibility of a leadless, ultrasound-based technology (the WiCS®-LV system) for endocardial left ventricular pacing. New tools have been developed for endocardial cardiac resynchronization therapy device implantation by interatrial septal puncture via subclavian access71 (Figure 4). Finally, the Mediguide electromagnetic tracking system drastically reduces fluoroscopy time.72
CONFLICTS OF INTERESTNone declared.