In Spain, intra-aortic balloon pump (IABP) has been used frequently as a bridge to urgent heart transplant (HT). We sought to analyze the clinical outcomes of this strategy.
MethodsWe conducted a case-by-case, retrospective review of clinical records of 281 adult patients listed for urgent HT under IABP support in 16 Spanish institutions from 2010 to 2015. Pre- and post-transplant survival and adverse clinical events were analyzed.
ResultsA total of 194 (69%, 95%CI, 63.3-74.4) patients were transplanted and 20 (7.1%, 95%CI, 4.4-10.8) died during a mean period of IABP support of 10.9±9.7 days. IABP support was withdrawn before an organ became available in 32 (11.4%) patients. Thirty-five (12.5%, 95%CI, 8.8-16.9) patients transitioned from IABP to full-support mechanical devices. Mean urgent waiting list time increased from 5.9±6.3 days in 2010 to 15±11.7 days in 2015 (P=.001). Post-transplant survival rates at 30-days, 1-year, and 5-years were 88.1% (95%CI, 85.7-90.5), 76% (95%CI, 72.9-79.1), and 67.8% (95%CI, 63.7-71.9), respectively. The incidence rate of major adverse clinical outcomes—device dysfunction, stroke, bleeding or infection—during IABP support was 26 (95%CI, 20.6-32.4) episodes per 1000 patient-days. The incidence rate of IABP explantation due to complications was 7.2 (95%CI, 4.5-10.8) cases per 1000 patient-days.
ConclusionsIn a setting of short waiting list times, IABP can be used to bridge candidates to urgent HT with acceptable postoperative results, but there were significant rates of adverse clinical events during support.
Keywords
Worldwide, intra-aortic balloon pump (IABP) remains as the most frequently used device to provide mechanical circulatory support (MCS) to patients with cardiogenic shock.1 As IABP is intended for partial, temporary support, it is unfrequently used as a direct bridge to heart transplant (HT). However, in the setting of the well-organized Spanish organ donor allocation system,2 which ensures short waiting times for candidates listed with urgent priority, IABP has been used frequently for this purpose.3
Previously published data regarding the results of IABP support as a direct bridge to HT are scarce. Preoperative use of IABP is a risk factor for post-transplant mortality according to an American registry-derived prognostic score4; however, a focused European single-center study found similar post-transplant survival in HT candidates bridged on IABP support compared with medically managed HT candidates.5 To the best of our knowledge, there are a lack of comprehensive data on waiting list mortality and clinical complications associated with IABP support in this bridge-to-transplant scenario.
We aimed to study in a systematic manner the pre- and post-transplant clinical outcomes of patients supported with an IABP with a primary intention of bridge-to-transplant in Spain in a recent era. To fulfil this objective, we analyzed the clinical information recorded in a nationwide registry.
METHODSStudy DescriptionThe ASIS-TC (Empleo de los dispositivos de asistencia circulatoria de corta duración como puente a trasplante cardiaco urgente en España) study was a retrospective, multicenter, registry that included all patients aged 18 years or older who were listed from 1 January 2010 to 31 December 2015 for first, single-organ, urgent HT within the Spanish national network for organ sharing—known as the Organización Nacional de Trasplantes—while being supported with short-term mechanical devices—IABP, extracorporeal membrane oxygenation or temporary ventricular assist devices (VAD). All 16 adult HT centers in the country participated in the registry.
The study protocol was approved by the Committee for Ethics in Clinical Investigation of the Autonomous Community of Galicia, and was ratified by the institutional review boards of participating hospitals.
During the study period, the highest waiting list priority level for HT within the Spanish organ donor allocation system—known as urgency status 0—was reserved exclusively for patients listed under extracorporeal membrane oxygenators or temporary VADs, or for patients with malfunctioning durable VADs. Status 0 conferred nationwide priority for receiving the first suitable organ donor available in the system. A recent study has reported the clinical outcomes of status 0 candidates.6
Candidates listed for HT under IABP support were included in a lower level of priority—known as urgency status 1—, which itself conferred advantage over medically managed candidates listed in the status 2 level—ie, the elective or nonurgent level— to receive a suitable cardiac donor, provided that no status 0 candidate could benefit from it. Before June 2014, priority of status 1 candidates over status 2 candidates applied to any organ retrieved within the whole nation, but beyond this date only to organs retrieved within the referral area of the transplant center where the candidate was hospitalized.
In this study, we report the clinical outcomes of patients listed for HT on IABP therapy as their unique MCS device, and therefore classified as urgency status 1. In the case of patients who were listed for HT as status 1 more than once during the study period, only the most recent episode of listing was included. The decision to implant an IABP and, subsequently, to include the patient on the waiting list for HT as urgency status 1 was adopted by each transplant team according to local protocols and clinical experience, but was not based on a prespecified protocol defined for the study.
Baseline clinical characteristics, outcomes and complications of IABP support, waiting times and post-transplant outcomes were analyzed. Specific definitions of study outcomes are presented in the .
Statistical AnalysisContinuous variables are presented as mean±standard deviation and categorical variables are presented as proportions. The Student t test and chi-squared test were used for statistical comparisons among groups, as required. The Clopper-Pearson exact method was used to estimate 95% confidence intervals (95%CI) of incidence rates of study outcomes. One-way analysis of variance with a lineal polynomial contrast was used to analyze the trend of waiting list times for HT over the study period.
Post-listing and post-transplant survival curves were depicted by means of the Kaplan-Meier method, and compared by means of the log-rank test. Multivariable Coxs regression was used to estimate the hazard ratio for 1-year mortality after urgent listing in patients with Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles 1-2 vs 3-4, as adjusted by age, sex, and the etiology of heart failure—ischemic or nonischemic. Statistical significance was set as a P value <.05 for all comparisons. Statistical analyses were performed with SPSS 20 and Epidat 4.1.
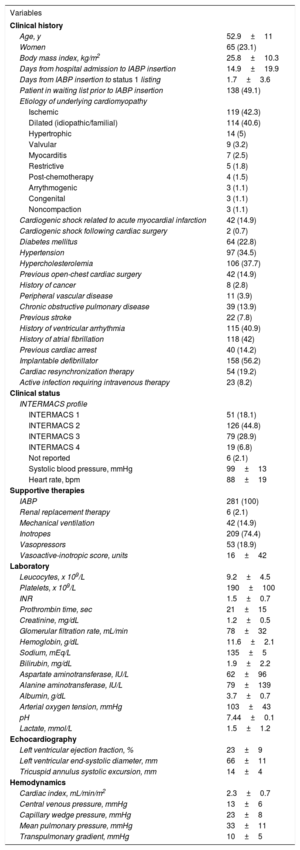
RESULTSPatientsFrom January 2010 to December 2015, 281 patients were listed for HT under IABP support, and therefore had a priority level of urgency status 1, in 16 Spanish hospitals. A total of 138 (49.1%) of these candidates had been already included in the waiting list for HT before IABP implantation, and had been upgraded to urgency status 1 after insertion of the device. Three patients were listed for status 1 HT under IABP support on more than 1 occasion during the study period.
An IABP was inserted through the femoral artery in all patients except 2 (0.7%), in which a brachial access was selected. The calibre of the device was 7-Fr in 89 (32%) patients, 8-Fr in 159 (57%) patients, and 9-Fr in 23 (8%) patients; this information was not reported in 10 patients. Mean time elapsed from IABP implantation to status 1 listing was 1.7±3.6 days.
Table 1 shows the most relevant baseline clinical characteristics of study patients, as considered at the time of status 1 listing.
Baseline Clinical Characteristics of Study Patients, at the Time of status 1 Listing
| Variables | |
|---|---|
| Clinical history | |
| Age, y | 52.9±11 |
| Women | 65 (23.1) |
| Body mass index, kg/m2 | 25.8±10.3 |
| Days from hospital admission to IABP insertion | 14.9±19.9 |
| Days from IABP insertion to status 1 listing | 1.7±3.6 |
| Patient in waiting list prior to IABP insertion | 138 (49.1) |
| Etiology of underlying cardiomyopathy | |
| Ischemic | 119 (42.3) |
| Dilated (idiopathic/familial) | 114 (40.6) |
| Hypertrophic | 14 (5) |
| Valvular | 9 (3.2) |
| Myocarditis | 7 (2.5) |
| Restrictive | 5 (1.8) |
| Post-chemotherapy | 4 (1.5) |
| Arrythmogenic | 3 (1.1) |
| Congenital | 3 (1.1) |
| Noncompaction | 3 (1.1) |
| Cardiogenic shock related to acute myocardial infarction | 42 (14.9) |
| Cardiogenic shock following cardiac surgery | 2 (0.7) |
| Diabetes mellitus | 64 (22.8) |
| Hypertension | 97 (34.5) |
| Hypercholesterolemia | 106 (37.7) |
| Previous open-chest cardiac surgery | 42 (14.9) |
| History of cancer | 8 (2.8) |
| Peripheral vascular disease | 11 (3.9) |
| Chronic obstructive pulmonary disease | 39 (13.9) |
| Previous stroke | 22 (7.8) |
| History of ventricular arrhythmia | 115 (40.9) |
| History of atrial fibrillation | 118 (42) |
| Previous cardiac arrest | 40 (14.2) |
| Implantable defibrillator | 158 (56.2) |
| Cardiac resynchronization therapy | 54 (19.2) |
| Active infection requiring intravenous therapy | 23 (8.2) |
| Clinical status | |
| INTERMACS profile | |
| INTERMACS 1 | 51 (18.1) |
| INTERMACS 2 | 126 (44.8) |
| INTERMACS 3 | 79 (28.9) |
| INTERMACS 4 | 19 (6.8) |
| Not reported | 6 (2.1) |
| Systolic blood pressure, mmHg | 99±13 |
| Heart rate, bpm | 88±19 |
| Supportive therapies | |
| IABP | 281 (100) |
| Renal replacement therapy | 6 (2.1) |
| Mechanical ventilation | 42 (14.9) |
| Inotropes | 209 (74.4) |
| Vasopressors | 53 (18.9) |
| Vasoactive-inotropic score, units | 16±42 |
| Laboratory | |
| Leucocytes, x 109/L | 9.2±4.5 |
| Platelets, x 109/L | 190±100 |
| INR | 1.5±0.7 |
| Prothrombin time, sec | 21±15 |
| Creatinine, mg/dL | 1.2±0.5 |
| Glomerular filtration rate, mL/min | 78±32 |
| Hemoglobin, g/dL | 11.6±2.1 |
| Sodium, mEq/L | 135±5 |
| Bilirubin, mg/dL | 1.9±2.2 |
| Aspartate aminotransferase, IU/L | 62±96 |
| Alanine aminotransferase, IU/L | 79±139 |
| Albumin, g/dL | 3.7±0.7 |
| Arterial oxygen tension, mmHg | 103±43 |
| pH | 7.44±0.1 |
| Lactate, mmol/L | 1.5±1.2 |
| Echocardiography | |
| Left ventricular ejection fraction, % | 23±9 |
| Left ventricular end-systolic diameter, mm | 66±11 |
| Tricuspid annulus systolic excursion, mm | 14±4 |
| Hemodynamics | |
| Cardiac index, mL/min/m2 | 2.3±0.7 |
| Central venous pressure, mmHg | 13±6 |
| Capillary wedge pressure, mmHg | 23±8 |
| Mean pulmonary pressure, mmHg | 33±11 |
| Transpulmonary gradient, mmHg | 10±5 |
IABP, intra-aortic balloon pump; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support.
Values are expressed as mean±standard deviation or No. (%).
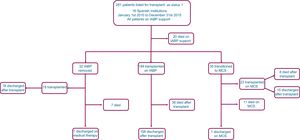
Mean duration of IABP support was 10.9±9.7 days (range, 0-58 days). During this period, programmed—ie, not due to complications—device replacement was performed once in 5 patients and twice in 2 patients. A flow chart of study patients and outcomes of IABP support is presented in Figure 1.
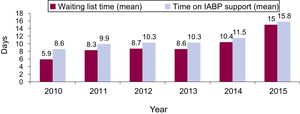
Overall, 194 (69%, 95%CI, 63.3-74.4) patients were transplanted and 20 (7.1%, 95%CI, 4.4-10.8) patients died during IABP support. Mean waiting time for HT was 9.6±10 days, increasing steadily over the study period, from 5.9±6.3 days in 2010 to 15±11.7 days in 2015 (Figure 2, P for lineal trend=.001).
Thirty-five (12.5%, 95%CI, 8.8-16.9) patients transitioned from IABP to full-support mechanical devices before an organ donor became available. Devices implanted were venoarterial extracorporeal membrane oxygenators (n=14), Levitronix Centrimag (n=13), Impella Recover (n=4), Abiomed BVS 5000 (n=3), and BerlinHeart Excor (n=1). Among these individuals, 11 patients died during MCS and 23 were subsequently transplanted as status 0 candidates—8 died during the early postoperative period after HT. One patient who underwent BerlinHeart Excor implantation was discharged from hospital on this device and was successfully transplanted 3 months later.
Intra-aortic balloon pump support was withdrawn before an organ donor became available in 32 (11.4%, 95%CI, 7.9-15.7) patients, who were subsequently managed medically. The reasons for cessation of IABP support were complications in 19 patients, clinical improvement in 10 patients, and futility in 3 patients. During their in-hospital stay after the cessation of IABP support, 18 patients underwent HT—all survived surgery—and 7 patients died without having been transplanted.
Overall, 235 (83.6%, 95%CI, 78.9-87.8) patients underwent HT during the in-hospital follow-up period after status 1 listing. Thirty-nine (13.9%, 95%CI, 10.1-18.5) patients died during hospital admission without having been transplanted.
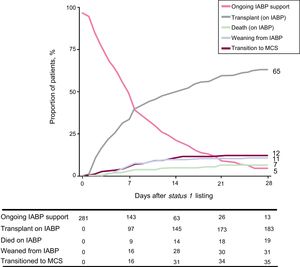
Figure 3 represents the occurrence of the competing events HT during IABP support, death during IABP support, transition to full-support mechanical devices, or IABP removal (and transition to medical management) in the study population over a 28-day follow-up period after status 1 listing.
Depiction of the competing outcomes analysis for death, weaning from IABP support, transition to full-support mechanical devices or transplantation during a 28-day follow-up period after status 1 listing. At any given time point, the sum of the proportion of patients experiencing each outcome equals 100%. IABP, intra-aortic balloon pump; MCS, mechanical circulatory support.
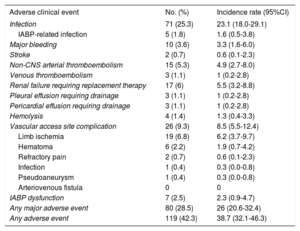
The incidence rate of adverse clinical events during IABP support was 38.7 (95%CI, 32.1-46.3) episodes per 1000 patient-days; the incidence rate of major adverse clinical events–major bleeding, stroke, infection or IABP dysfunction–was 26.0 (95%CI, 20.6-32.4) episodes per 1000 patient-days. Table 2 shows the incidence rate of all individual adverse clinical events reported in the study.
Adverse Clinical Events During Intra-aortic Balloon Pump Support
| Adverse clinical event | No. (%) | Incidence rate (95%CI) |
|---|---|---|
| Infection | 71 (25.3) | 23.1 (18.0-29.1) |
| IABP-related infection | 5 (1.8) | 1.6 (0.5-3.8) |
| Major bleeding | 10 (3.6) | 3.3 (1.6-6.0) |
| Stroke | 2 (0.7) | 0.6 (0.1-2.3) |
| Non-CNS arterial thromboembolism | 15 (5.3) | 4.9 (2.7-8.0) |
| Venous thromboembolism | 3 (1.1) | 1 (0.2-2.8) |
| Renal failure requiring replacement therapy | 17 (6) | 5.5 (3.2-8.8) |
| Pleural effusion requiring drainage | 3 (1.1) | 1 (0.2-2.8) |
| Pericardial effusion requiring drainage | 3 (1.1) | 1 (0.2-2.8) |
| Hemolysis | 4 (1.4) | 1.3 (0.4-3.3) |
| Vascular access site complication | 26 (9.3) | 8.5 (5.5-12.4) |
| Limb ischemia | 19 (6.8) | 6.2 (3.7-9.7) |
| Hematoma | 6 (2.2) | 1.9 (0.7-4.2) |
| Refractory pain | 2 (0.7) | 0.6 (0.1-2.3) |
| Infection | 1 (0.4) | 0.3 (0.0-0.8) |
| Pseudoaneurysm | 1 (0.4) | 0.3 (0.0-0.8) |
| Arteriovenous fistula | 0 | 0 |
| IABP dysfunction | 7 (2.5) | 2.3 (0.9-4.7) |
| Any major adverse event | 80 (28.5) | 26 (20.6-32.4) |
| Any adverse event | 119 (42.3) | 38.7 (32.1-46.3) |
95%CI, 95% confidence interval; CNS, central nervous system; IABP, intra-aortic balloon pump.
Incidence rate is expressed in number of patients having an event per 1000 patients-day of support.
Intra-aortic balloon pump explantation due to device-related complications was performed in 22 (7.8%) patients before an organ became available; in 3 of these individuals, a new IABP was reinserted. Causes for IABP explantation were ischemia/arterial thromboembolism (n=11), device dysfunction (n=5), infection (n=4), and refractory pain (n=2). The incidence rate of IABP explantation due to device-related complications was 7.2 (95%CI, 4.5-10.8) cases per 1000 patient-days.
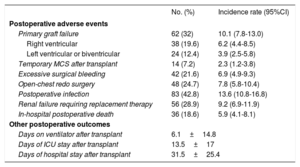
Post-transplant OutcomesAmong 194 patients who underwent urgent HT while on IABP support, 36 (18.6%, 95%CI, 13.3-24.7) died within the in-hospital postoperative period. Table 3 shows the incidence rates of other relevant in-hospital postoperative outcomes after HT. Mean cold ischemic time of these procedures was 213±52min. Mean age of the donors was 42±12.6 years. Donors aged ≥ 45 years and donors with ischemic time ≥ 240minutes were used in 93 (47.9%) and 66 (34%) recipients, respectively.
In-hospital Postoperative Outcomes After Heart Transplant in 194 Patients Bridged Under Intra-aortic Balloon Pump Support
| No. (%) | Incidence rate (95%CI) | |
|---|---|---|
| Postoperative adverse events | ||
| Primary graft failure | 62 (32) | 10.1 (7.8-13.0) |
| Right ventricular | 38 (19.6) | 6.2 (4.4-8.5) |
| Left ventricular or biventricular | 24 (12.4) | 3.9 (2.5-5.8) |
| Temporary MCS after transplant | 14 (7.2) | 2.3 (1.2-3.8) |
| Excessive surgical bleeding | 42 (21.6) | 6.9 (4.9-9.3) |
| Open-chest redo surgery | 48 (24.7) | 7.8 (5.8-10.4) |
| Postoperative infection | 83 (42.8) | 13.6 (10.8-16.8) |
| Renal failure requiring replacement therapy | 56 (28.9) | 9.2 (6.9-11.9) |
| In-hospital postoperative death | 36 (18.6) | 5.9 (4.1-8.1) |
| Other postoperative outcomes | ||
| Days on ventilator after transplant | 6.1±14.8 | |
| Days of ICU stay after transplant | 13.5±17 | |
| Days of hospital stay after transplant | 31.5±25.4 | |
95%CI, 95% confidence interval; ICU, intensive care unit; MCS, mechanical circulatory support.
Unless otherwise indicated, data are expressed as No. (%) or mean ± standard deviation.
Incidence rate is expressed in number of patients having an event per 1000 patients-day of postoperative stay after transplant.
Causes of in-hospital postoperative death after HT were early graft failure (n=17), infection (n=7), surgical bleeding (n=5), rejection (n=3), sudden death (n=1), and nonspecified multiorgan failure (n=3).
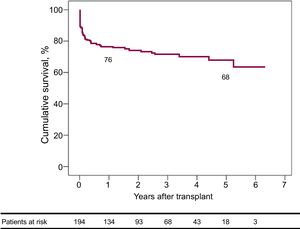
By means of the Kaplan-Meier method, estimated 30-day, 1-year, and 5-year survival rates after HT were 88.1% (95%CI, 85.7-90.5), 76% (95%CI, 72.9-79.1), and 67.8% (95%CI, 63.7-71.9), respectively (Figure 4).
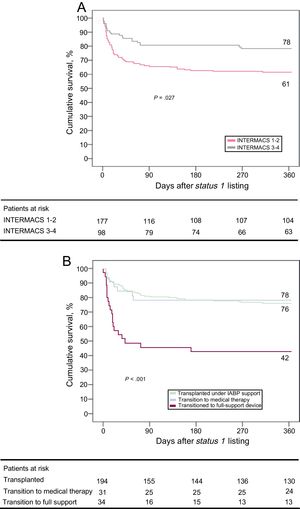
Overall Survival After Status 1 ListingOverall 1-year survival after status 1 listing, considering both on-support and either post-transplant (if transplanted) or postsupport (if not transplanted) periods, was 66.7% (95%CI, 63.9-69.5). Adjusted hazard ratio for 1-year all-cause mortality after status 1 listing in patients with INTERMACS profiles 1-2 vs 3-4, as adjusted by age, sex and the etiology of heart failure was 2.17 (95%CI, 1.32-3.58, P=.005, Figure 5A). INTERMACS 1-2 candidates showed higher rates of transition to full-support devices (16.9% vs 4.1%; P=.002) and lower rates of transplantation (65% vs 78.6%; P=.019) during IABP support than INTERMACS 3-4 candidates.
A: survival over the first year after status 1 listing according to INTERMACS status. B: survival over the first year after status 1 listing according to the endpoint of intra-aortic balloon pump support. IABP, intra-aortic balloon pump; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support.
One-year survival after status 1 listing varied significantly according to the clinical endpoint of IABP support (Figure 5B, P=.001). Patients who transitioned from IABP to full-support mechanical devices showed the worst outcomes, with an estimated 1-year survival after status 1 listing of 42.9% (95%CI, 34.5-51.3). Estimated 1-year survival after status 1 listing was 78.1% (95%CI, 70.8-85.4) in patients in whom IABP support was stopped and who were subsequently managed medically; this was comparable to survival of candidates transplanted directly on IABP support. Specific 1-year post-transplant survival curves in these 3 subgroups of patients are presented in the .
DISCUSSIONIn this nationwide registry, we studied the pre- and post-transplant clinical outcomes of 281 adult patients listed for urgent HT under IABP support in 16 Spanish institutions from 2010 to 2015. Roughly more than two-thirds of them received a donor heart under IABP support within a mean waiting period of ∼10 days. Thirty-day, 1-year and 5-year post-transplant survival rates were ∼88%, ∼76% and ∼68%, respectively. Worse outcomes were observed among candidates who required transition from IABP to full-support MCS devices due to progressive hemodynamic impairment while awaiting transplantation. In this subgroup, 1-year survival after urgent listing dropped to ∼43%.
Worldwide, IABP is the most widely available method to provide MCS. The main advantage of this device is its easy percutaneous implantation, which allows an immediate, bedside, start of support. IABP therapy moderately decreases systemic vascular resistance and moderately increases cardiac output up to ∼1 litre per minute, provided that the failing left ventricle maintains some contractility that allows counterpulsation.7 These hemodynamic effects increase peripheral perfusion, ameliorating end-organ damage.8 In patients with ischemic heart disease, diastolic augmentation of aortic blood pressure mediated by IABP inflation significantly increases coronary blood flow.9 In high-risk patients undergoing coronary artery bypass grafting, preoperative initiation of IABP support significantly reduces the risk of postoperative renal failure.10
The hemodynamic improvement conferred by IABP therapy is usually sufficient for the initial stabilization of many patients with advanced HF and signs of low cardiac output, especially in the setting of ischemic heart disease, as a bridge to decision or recovery. However, IABP is unfrequently used as a direct bridge to HT, mainly due to 2 reasons. First, the partial support provided by the device is often insufficient for patients with profound hemodynamic compromise, or for those with right ventricular failure. Indeed, recent data suggest that IABP therapy does not impact survival in patients with cardiogenic shock complicating an acute myocardial infarction.11 Second, IABP is conceptually intended as a short-term therapy, so its usefulness as a bridge to HT is jeopardized by the chance that the patient has to get a suitable donor in the first few days after the start of support. IABP implantation through a brachial access has been proposed as a safe alternative that allows more prolonged support than the classic femoral insertion12; however, this approach is rarely used in current practice.
It is remarkable that even in a setting of short waiting times for HT, as is the case of the efficient Spanish organ donor sharing network,1 almost one-third of the studied patients was not allocated a donor heart during IABP support. Of note, survival was significantly impaired in candidates listed with INTERMACS profiles 1 and 2,13 probably indicating that IABP was an insufficient support for many of them. Prognosis was especially ominous in candidates who transitioned from IABP to full-support MCS—in most cases with temporary devices—due to progressive clinical deterioration while awaiting HT, even though a significant proportion of them upgraded to the top level of waiting list priority (urgency status 0) and were subsequently transplanted.
Post-transplant outcomes of patients who could be effectively bridged to HT on IABP support were acceptable, as they were comparable to those reported from the whole historical cohort of Spanish HT recipients.14 The incidence of early postoperative complications and causes of death were, in general terms, similar to expected, with the remarkable exception of an abnormally high rate of primary graft failure, which might be attributable to a frequent use of aged donors with long ischemic times; however, it must be noticed that local investigators were responsible for the recording of adverse clinical events, so a positive observation bias cannot be ruled out totally.
Although preoperative IABP support has been identified as a risk factor for post-transplant mortality in a multivariable model derived from the United Organ Network for Organ Sharing registry,4 an independent negative impact of IABP on post-transplant outcomes was not confirmed by a focused European single-center study.5 From a clinical point of view, it seems unlike that the device itself significantly impacts the risk of transplant surgery; rather, we feel that discrepancies among reported data more probably reflect a heterogeneous risk profile of studied participants. Indeed, previous literature suggests that the clinical status of the recipient, rather than the type of support used, is probably the strongest predictor of postoperative outcomes following HT.13
Another notable feature of our study is that nearly one-half of the patients listed as status 1 under IABP support were not newly listed candidates, but had been previously listed in the nonurgent level. Given the increasing scarcity of donors and waiting times for HT, the semielective implantation of a durable VAD as bridge to HT in early declining or, even stable, inotrope-dependent patients emerges as the most reasonable strategy to break the vicious circle that is now favoring a progressive increase in the proportion of donor hearts that are destined annually in our country to urgent candidates.14 Recent data from the International Society for Heart and Lung Transplantation registry suggest a protective effect of preoperative continuous-flow VAD support on post-transplant outcomes, which is probably due to the beneficial effects of the device on end-organ function, frailty, and nutritional status.15 This argument was reinforced by a recent single-center American study,16 which showed better clinical outcomes in candidates who transitioned from short-term to long-term devices and were then subsequently transplanted, compared with candidates who underwent HT directly while on temporary MCS.
In view of this situation, Spanish health care authorities have recently remodelled the priority listing criteria for HT candidates. Since 2017, IABP support is no longer considered an urgent indication, and the criteria required for urgent listing in patients supported with extracorporeal membrane oxygenators of temporary VADs are more restrictive.17
In our cohort, the overall daily rate of adverse clinical events associated with IABP support approached ∼4%. This incidence rate is lower than the rate of adverse events reported for candidates treated with temporary MCS,6 but even so, seems higher than that observed in carriers of durable VADs.18 Most adverse clinical events recorded in the study, like infection, bleeding, or thromboembolic complications may be considered, to a certain extent, as inherent to the critical clinical status of studied patients, but in some cases they also could be favored by IABP therapy.
The cumulative rate of IABP exchange due to complications directly attributable to the device, such as limb ischemia, mechanical dysfunction, or refractory pain, was 7.8% during the whole period of support; other studies have shown a rate of complications related to IABP support ranging from 2.6% to 13%.19,20 These data show how IABP therapy, despite its apparent simplicity and wide availability, is not at all an innocuous modality.
LimitationsThis study has a few limitations. First, as an observational study, it is exposed to potential information, selection, observation and confusion biases. Second, the nature of this investigation is essentially descriptive, rather than analytic; the lack of a parallel control group of matched medically managed HT candidates prevented us from drawing consistent conclusions about an independent effect of IABP on pre- or post-transplant survival. Third, the study population was heterogeneous, as patients from 16 different institutions with variable center-specific protocols and clinical experience were included. Fourth, all patients were treated in the setting of the Spanish national organ sharing network, which is historically characterized by extremely short waiting times for urgent HT indications; consequently, the external validity of our observations is questionable, and our results might not be directly extrapolated to other countries. Finally, it must be recognized that the study focused on a recent—but, to some extent, also past—period of the evolution of the field; in view of the recent changes in clinical criteria required for urgent HT listing in Spain, it is presumable that the use of IABP as a direct bridge to HT will become less frequent in future years.
CONCLUSIONSIntra-aortic balloon pump remains as a widely available method to provide initial circulatory support to critically ill HT candidates. For those who achieve clinical stabilization, IABP can be used as a direct bridge to urgent HT, provided that expected waiting times are short, with acceptable post-transplant outcomes. However, for those who experience profound hemodynamic impairment despite IABP therapy, early implantation of a full-support mechanical device should be considered.
Despite its apparent simplicity, IABP therapy is not an innocuous technique, as it is associated with a significant risk of adverse clinical events as limb ischemia, thromboembolism, device dysfunction, and infection.
In future years, increasing waiting list times and more restrictive indications for urgent HT will probably jeopardize the usefulness of IABP support as a direct bridge to HT in our country.
FUNDINGE. Barge-Caballero received a research grant from the Fundación Mutua Madrileña, Spain (XI Annual announcement, year 2014), which supplied funding for the present study.
Some of the authors of this manuscript are members of the CIBERCV (Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares), Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness.
CONFLICTS OF INTERESTE. Barge-Caballero received an academic grant from St. Jude Medical, not related to this investigation.
- –
The use of IABP as a direct bridge to HT is conditioned by the fact that a suitable organ donor must become available for the patient within a few days after the start of support.
- –
Unlike other countries, short waiting times for urgently listed candidates have historically favored the use of IABP as a direct bridge to HT in Spain.
- –
Our study provides a unique systematic description of pretransplant and post-transplant outcomes in patients supported with IABP with a primary intention of bridge-to-transplant in our country.
- –
The study shows how IABP was a feasible option for bridging Spanish patients to urgent HT in the past, providing reasonable postoperative outcomes. However, increasing waiting list times and more restrictive indications for urgent HT will probably jeopardize the usefulness of this strategy in future years.
The authors are grateful to the Organización Nacional de Trasplantes for its support to this investigation.