The transradial approach is associated with a reduction in vascular access-related complications after primary percutaneous coronary interventions. The purpose of this study was to examine the feasibility of the routine use of transradial access in primary angioplasty and to evaluate how it affects subgroups with less favorable characteristics.
MethodsWe analyzed 1029 consecutive patients with an ST-segment elevation acute coronary syndrome treated with primary angioplasty.
ResultsTransradial access was the primary approach in 93.1% of the patients. The success rate of primary angioplasty was 95.9%, and 87.6% of the patients were event-free 30 days after the procedure. Crossover was required in 3.0% of the patients with primary transradial access, and this rate remained stable over the years. Predictors of the need for crossover were age older than 75 years (odds ratio=2.50, 95% confidence interval, 1.09–5.71; P=.03) and a history of ischemic heart disease (odds ratio=2.65; 95% confidence interval, 1.12–6.24; P=.02). Primary transfemoral access use was higher in women older than 75 years. Use of the transradial approach in this subgroup did not affect reperfusion time or the success of angioplasty, although there was a greater need for crossover (10.9% vs 2.6%; P=.006). Among patients in cardiogenic shock, the transradial approach was used in 51.5%; reperfusion times and angioplasty success rates were similar to those obtained with transfemoral access, but there was a greater need for crossover.
ConclusionsTransradial access can be used safely and effectively in most primary angioplasty procedures. In older women and in patients in cardiogenic shock, there is a higher crossover requirement, with no detriment to reperfusion time.
Keywords
Primary percutaneous coronary intervention (PPCI) is the preferred treatment for patients with ST-segment acute coronary syndrome (STEACS). Systematic use of this procedure improves the outcome of reperfusion in these patients, while bringing to light complications related to vascular access.1,2 There is sufficient evidence that patients with periprocedural bleeding have an unfavorable prognosis.3,4
Although these complications are generally uncommon, several studies published since 2003 have shown that transradial/ulnar access (TRUA) is associated with a lower risk of developing such complications than transfemoral access (TFA), with no detriment to reperfusion time.5–11 These studies, which are limited by the small number of patients included and by selection bias, have generated an ongoing debate in the scientific community since the first angioplasty procedure using radial access was reported in 1993 by Kiemeneij and Laarman.12
The results of the largest study comparing the surgical approaches used in PPCI were published in 2011.13 Despite the growing evidence on this issue, there remains considerable controversy on the routine use of TRUA, based on the idea that this approach could affect the success of angioplasty and reperfusion time in specific patient groups. The interventional cardiology unit in our center has wide experience in TRUA (more than 90% of all angioplasties performed). The aim of this study was to evaluate the feasibility of the routine use of TRUA for PPCI in a high-volume center and to analyze its effect in patient subgroups with less favorable characteristics.
METHODSPatients and ProcedureThe analysis included all consecutive patients with an STEACS treated by PPCI in Hospital Universitario Puerta de Hierro de Majadahonda (Madrid, Spain) between January 2005 and December 2011. In our center, 85% of the patients with STEACS receive PPCI treatment.14 PPCI was indicated in patients with symptoms of angina of less than 12 h’ duration and ST-segment elevation greater than 0.1 mV in at least 2 contiguous leads on electrocardiography. Patients received dual antiplatelet therapy with a loading dose of 300 mg of acetylsalicylic acid and 600 mg of clopidogrel. Since 2011, patients younger than 75 years weighing more than 60 kg and with no history of previous stroke have received a loading dose of 60 mg of prasugrel, with a subsequent regimen of 10 mg daily.15 In addition, during the procedure, an initial dose of 5000 IU of sodium heparin was administered, followed by 1000 IU for each additional 30 min’ duration, as well as glycoprotein IIb/IIIa inhibitors, which in most patients was 2 intravenous bolus doses of eptifibatide 180 μg/kg, 10min apart, followed by infusion of 2 μg/kg/min for 12 h.
The procedures were carried out by 5 interventional cardiologists highly experienced in performing TRUA. The primary access route was at the discretion of the operator. In most patients, the right radial artery was used, with a 6 Fr introducer. A spasmolytic cocktail containing verapamil was routinely used to avoid radial spasm. The introducer was withdrawn in the catheterization laboratory and various devices were used for TRUA hemostasis (TR-Band®, D-Stat®, and conventional access).
Definitions- •
Primary access: the first vascular approach attempted, regardless of whether or not it was successful.
- •
TRUA: vascular access obtained in the wrist area, usually the right radial artery, and less often, the left radial artery or ulnar artery.
- •
Crossover: change of vascular access when the procedure could not be carried out through the primary access.
- •
Successful PPCI: angiographically-proven residual stenosis of less than 50% and TIMI flow greater than or equal to 2, and no death, reinfarction, acute or subacute thrombosis, or need for a new percutaneous or surgical revascularization procedure in the artery causing the infarction.
- •
Needle-guidewire time: time from the first radial puncture to passage of the angioplasty guidewire through the obstruction, in minutes.
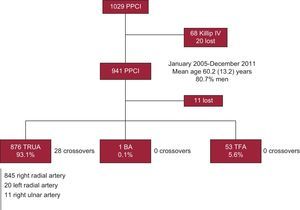
During catheterization, clinical cardiologists prospectively collected demographic data, the patients’ baseline characteristics, and the procedure-related characteristics, and entered the information in the PPCI database of our center. A total of 1029 PPCIs were performed during the study period. A separate analysis was performed in 68 patients (6.6%) in cardiogenic shock (see “Special Subgroups: Patients in Cardiogenic Shock”), and 20 patients (1.9%) were excluded because their clinical status at presentation was unknown. Hence, 941 PPCI procedures formed the nucleus of our study (Fig. 1). Data were analyzed retrospectively at completion of the recruitment period.
- •
Primary aim: To evaluate the feasibility of routine use of TRUA in PPCI, analyzing the crossover rate and the procedure-related variables (fluoroscopy time, needle-guidewire time, contrast volume, and angioplasty success rate).
- •
Secondary Aims:
- –
To identify the clinical and procedure-related variables associated with a greater need for crossover or primary use of TFA. To characterize a less favorable patient subgroup using the above-defined variables.
- –
To evaluate the effect of the use of TRUA in this less favorable subgroup, by analyzing the angioplasty success rate and the above-proposed procedure-related variables.
- –
Qualitative variables were analyzed with the chi-square test for parametric data and the Fisher exact test for nonparametric data, and are expressed as rates or percentages. Quantitative variables were analyzed with the Student t test or analysis of variance for more than 2 measures, and are expressed as the mean (standard deviation). Survival is represented by Kaplan-Meier curves. Univariate and multivariate logistic regression analyses were used to identify the variables associated with a greater need for crossover or greater use of the femoral access. All tests were 2-tailed, and results were considered statistically significant at a P value of <.05. The statistical analysis was performed with SPSS (SPSS V.20.0 for Macintosh).
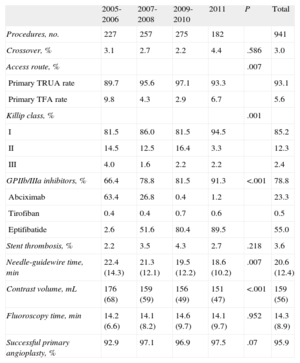
RESULTSStudy PopulationBetween January 2005 and December 2011, 941 PPCIs were performed. The mean age of the patients was 60.2 (13.2) years, and 80.7% were men. Between 2005 and 2011, the percentage of smokers increased from 64.7% to 74.7% (P=.009). The demographic and clinical characteristics of the study population are summarized in Table 1.
Demographic and Clinical Characteristics of the Population Studied
| 2005-2006 | 2007-2008 | 2009-2010 | 2011 | P | Total | |
| Procedures, no. | 227 | 257 | 275 | 182 | 941 | |
| Age, years | 61 (13) | 61 (13) | 59 (13) | 59 (14) | .05 | 60 (13) |
| Age≥75 years, % | 21.1 | 16.0 | 13.1 | 15.4 | .109 | 16.3 |
| Women, % | 19.8 | 22.2 | 18.5 | 15.4 | .359 | 19.3 |
| Weight, kg | 78 (13) | 79 (13) | 79 (14) | 80 (17) | .625 | 79 (14) |
| Height, cm | 167 (8) | 168 (9) | 169 (9) | 168 (12) | .613 | 168 (9) |
| BMI | 27 (4) | 28 (4) | 28 (4) | 30 (22) | .089 | 28 (11) |
| BSA, m2 | 1.8 (0.2) | 1.9 (0.2) | 1.9 (0.2) | 1.9 (0.2) | .53 | 1.9 (0.2) |
| HT, % | 48.5 | 47.1 | 46.2 | 41.2 | .493 | 46.1 |
| Diabetes mellitus, % | 23.3 | 18.7 | 17.1 | 17.0 | .278 | 19.1 |
| Dyslipidemia, % | 40.1 | 38.9 | 40.4 | 36.3 | .817 | 39.2 |
| Smoking, % | 64.7 | 70.4 | 77.8 | 74.7 | .009 | 72.1 |
| Previous ischemic heart disease, % | 12.8 | 12.8 | 14.5 | 13.2 | .927 | 13.4 |
BMI, body mass index; BSA, body surface area; HT, hypertension.
TRUA was the primary access route in 876 of 941 patients (93.1%; 845 in the right radial artery, 20 in the left radial artery, 11 in the right ulnar artery). The left brachial artery was used in only 1 patient (1/941; 0.1%) and the femoral artery in 53 of 941 cases (5.6%; 51 right and 2 left femoral artery). Information was not available in 11 patients (Fig. 1). Primary TRUA use significantly increased from the 2005-2006 period to 2011 (89.7% vs 93.3%; P=.007) and primary TFA use decreased (9.8% vs 6.7%; P=.007) (Table 2).
Procedure-related Characteristics in the Population Studied
| 2005-2006 | 2007-2008 | 2009-2010 | 2011 | P | Total | |
| Procedures, no. | 227 | 257 | 275 | 182 | 941 | |
| Crossover, % | 3.1 | 2.7 | 2.2 | 4.4 | .586 | 3.0 |
| Access route, % | .007 | |||||
| Primary TRUA rate | 89.7 | 95.6 | 97.1 | 93.3 | 93.1 | |
| Primary TFA rate | 9.8 | 4.3 | 2.9 | 6.7 | 5.6 | |
| Killip class, % | .001 | |||||
| I | 81.5 | 86.0 | 81.5 | 94.5 | 85.2 | |
| II | 14.5 | 12.5 | 16.4 | 3.3 | 12.3 | |
| III | 4.0 | 1.6 | 2.2 | 2.2 | 2.4 | |
| GPIIb/IIIa inhibitors, % | 66.4 | 78.8 | 81.5 | 91.3 | <.001 | 78.8 |
| Abciximab | 63.4 | 26.8 | 0.4 | 1.2 | 23.3 | |
| Tirofiban | 0.4 | 0.4 | 0.7 | 0.6 | 0.5 | |
| Eptifibatide | 2.6 | 51.6 | 80.4 | 89.5 | 55.0 | |
| Stent thrombosis, % | 2.2 | 3.5 | 4.3 | 2.7 | .218 | 3.6 |
| Needle-guidewire time, min | 22.4 (14.3) | 21.3 (12.1) | 19.5 (12.2) | 18.6 (10.2) | .007 | 20.6 (12.4) |
| Contrast volume, mL | 176 (68) | 159 (59) | 156 (49) | 151 (47) | <.001 | 159 (56) |
| Fluoroscopy time, min | 14.2 (6.6) | 14.1 (8.2) | 14.6 (9.7) | 14.1 (9.7) | .952 | 14.3 (8.9) |
| Successful primary angioplasty, % | 92.9 | 97.1 | 96.9 | 97.5 | .07 | 95.9 |
GPIIb/IIIa, glycoprotein IIb/IIIa; TFA, transfemoral access; TRUA, transradial/ulnar access.
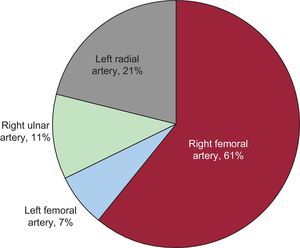
Crossover was required in 28 of 941 PPCIs, which yielded a crossover rate of 3.0%. All crossover cases occurred at the start of the procedure with TRUA (28 in the right radial artery). There were no significant differences in the crossover rate between the periods studied (3.1% in 2005-2006 vs 4.4% in 2011; P=.585). Among the 28 patients requiring a change of vascular access, the femoral artery was chosen in 19 (68%) (17 right femoral, 2 left femoral), the right ulnar artery (homolateral to the radial route at the start of the procedure) in 3 (11%), and the contralateral radial artery in the remaining 6 (21%) patients (Fig. 2). The most common reasons for crossover were difficulty canalizing the artery owing to a weak pulse (15/28 patients) and inability to advance the guidewire due to peripheral artery occlusion or tortuosity of the brachiocephalic trunk (6/28 patients). Only 2 PPCI procedures were performed on coronary bridges, 1 by left TRUA and the other by ATF; crossover was not required in either patient. On multivariate analysis, 2 factors were identified as predictive of the need for crossover: older than 75 (odds ratio [OR]=2.50; 95% confidence interval [95%CI], 1.09-5.71; P=.03) and a history of previous ischemic heart disease (OR=2.65; 95%CI, 1.12-6.24; P=.02).
During the procedure, the mean fluoroscopy time was 14.3 (8.9) min, needle-guidewire time was 20.6 (12.4) min, and contrast volume was 159 (56) mL. Between 2005-2006 and 2011, needle-guidewire time significantly decreased from 22.4 (14.3) min to 18.6 (10.2) min (P=.007) and contrast volume from 176 (68) mL to 151 (47) mL (P<.001), whereas fluoroscopy time remained stable (14.2 [6.6] min vs 14.1 [9.7] min; P=.952). In patients requiring crossover, fluoroscopy time (22.3 vs 14.1 min; P=.009), needle-guidewire time (38.1 vs 20.0 min; P<.001), and contrast volume (204 vs 158 mL; P<.001) were significantly higher than in the remaining patients.
The success rate of PPCI in our series was 95.9%, and it remained stable during the periods studied (2005-2006 vs 2011, 92.9% vs 97.5%; P=.07). In patients requiring crossover, the success rate was significantly lower (78.0% vs 96.4%; P=.02). Nonetheless, in the multivariate analysis, crossover requirement was not predictive of a lack of success of PPCI (OR=1.24; 95%CI, 0.58-2.77; P=.56).
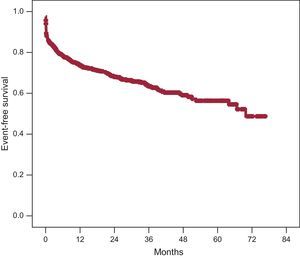
Survival was 97.7% at 30 days following PPCI, and 95.6% at 1 year. In the survival analysis, with a composite endpoint of events that included death, angina, reinfarction, new angioplasty, need for cardiac surgery, and heart failure, the event-free cumulative survival was 87.6% at 30 days after the procedure and 75.9% at 1 year (Fig. 3).
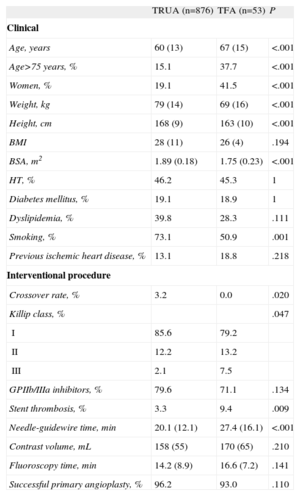
Primary Transfemoral AccessPrimary TFA was used in 53 (5.6%) of the 941 patients who underwent PPCI. Patients with primary TFA were older, there were more women, and body weight, height, and body surface area were lower than in patients who underwent primary TRUA. Furthermore, there were fewer patients with a history of smoking, and more cases of stent thrombosis and Killip III infarction (Table 3). On multivariate analysis, the predictors of a primary TFA use were age older than 75 years (OR=2.88; 95%CI; 1.36-6.07; P=.005) and female sex (OR=2.91; 95%CI; 1.39-6.09; P=.005).
Clinical and Procedure-related Differences According to the Primary Vascular Access
| TRUA (n=876) | TFA (n=53) | P | |
| Clinical | |||
| Age, years | 60 (13) | 67 (15) | <.001 |
| Age>75 years, % | 15.1 | 37.7 | <.001 |
| Women, % | 19.1 | 41.5 | <.001 |
| Weight, kg | 79 (14) | 69 (16) | <.001 |
| Height, cm | 168 (9) | 163 (10) | <.001 |
| BMI | 28 (11) | 26 (4) | .194 |
| BSA, m2 | 1.89 (0.18) | 1.75 (0.23) | <.001 |
| HT, % | 46.2 | 45.3 | 1 |
| Diabetes mellitus, % | 19.1 | 18.9 | 1 |
| Dyslipidemia, % | 39.8 | 28.3 | .111 |
| Smoking, % | 73.1 | 50.9 | .001 |
| Previous ischemic heart disease, % | 13.1 | 18.8 | .218 |
| Interventional procedure | |||
| Crossover rate, % | 3.2 | 0.0 | .020 |
| Killip class, % | .047 | ||
| I | 85.6 | 79.2 | |
| II | 12.2 | 13.2 | |
| III | 2.1 | 7.5 | |
| GPIIb/IIIa inhibitors, % | 79.6 | 71.1 | .134 |
| Stent thrombosis, % | 3.3 | 9.4 | .009 |
| Needle-guidewire time, min | 20.1 (12.1) | 27.4 (16.1) | <.001 |
| Contrast volume, mL | 158 (55) | 170 (65) | .210 |
| Fluoroscopy time, min | 14.2 (8.9) | 16.6 (7.2) | .141 |
| Successful primary angioplasty, % | 96.2 | 93.0 | .110 |
BMI, body mass index; BSA, body surface area; GPIIb/IIIa, glycoprotein IIb/IIIa; HT, hypertension; TFA, transfemoral access; TRUA, transradial/ulnar access.
The needle-guidewire time was greater in patients with TFA than in those with TRUA (27.4 [16.1] vs 20.1 [12.1] min; P<.001), whereas fluoroscopy time (16.6 [7.2] vs 14.2 [8.9] min; P=.141), and contrast volume (170 [65] vs 158 [55] mL; P=.210) showed no significant differences. There were no cases of crossover with TFA (0% vs 3.2%; P=.02) and the PPCI success rate was similar to that observed with TRUA (93.0% vs 96.2%; P=.110) (Table 3).
Identification of a Less Favorable Subgroup and Subgroup ResultsBased on the variables that led to the choice of a primary vascular access other than TRUA, and reinforced by the additional greater need for crossover in patients older than 75 years, we identified a patient profile that was less favorable for TRUA: women older than 75 years. The use of TRUA was lower in this subgroup than in the remaining patients but was still quite frequent (80% vs 95.5%; P=.001). In the subgroup analysis of women older than 75 years in whom TRUA was the primary approach used (n=55), there were no differences in the PPCI success rate relative to the remaining patients (90.0% vs 96.4%; P=.105) or in the time to reperfusion (needle-guidewire time, 22.0 [12.7] vs 20.0 [12.0] min; P=.243; fluoroscopy time, 14.8 [8.1] vs 14.1 [8.9] min; P=.669; or contrast volume, 158 [61] vs 158 [54] mL, P=.987), but there was a significant increase in the crossover rate (10.9% vs 2.6%; P=.006).
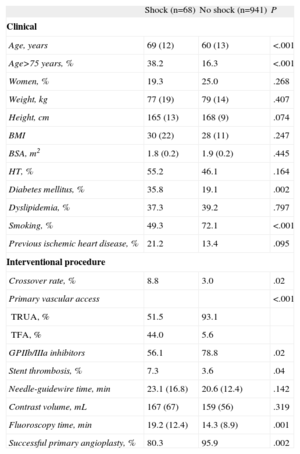
Special Subgroups: Patients in Cardiogenic ShockBetween 2005 and 2011, 68 patients (6.6%) underwent PPCI while in cardiogenic shock. The mean age of this subgroup was 69.2 (12.1) years and the percentage of patients older than 75 years was higher than in the remaining groups (38.2% vs 16.3%, P<.001). Furthermore, there was a higher rate of diabetes mellitus (35.8% vs 19.1%; P=.002) and fewer patients with a history of smoking (49.3% vs 72.1%; P<.001).
The primary approach was TRUA in 35 of 68 patients (51.5%), the brachial artery in 1 patient (1.5%), and the femoral approach in 30 of 68 patients (44.0%). In 2 of 68 patients (3.0%), this information was not available. The crossover rate in patients in shock was significantly higher than in the remaining patients (8.8 vs 3.0%; P=.02) and the PPCI success rate was lower (80.3% vs 95.9%; P=.002). In addition, glycoprotein IIb/IIIa inhibitors were used less frequently, and there was a lower rate of stent thrombosis (Table 4).
Clinical and Procedure-related Differences Between Patients in Cardiogenic Shock and the Remaining Series at the Time of Presentation
| Shock (n=68) | No shock (n=941) | P | |
| Clinical | |||
| Age, years | 69 (12) | 60 (13) | <.001 |
| Age>75 years, % | 38.2 | 16.3 | <.001 |
| Women, % | 19.3 | 25.0 | .268 |
| Weight, kg | 77 (19) | 79 (14) | .407 |
| Height, cm | 165 (13) | 168 (9) | .074 |
| BMI | 30 (22) | 28 (11) | .247 |
| BSA, m2 | 1.8 (0.2) | 1.9 (0.2) | .445 |
| HT, % | 55.2 | 46.1 | .164 |
| Diabetes mellitus, % | 35.8 | 19.1 | .002 |
| Dyslipidemia, % | 37.3 | 39.2 | .797 |
| Smoking, % | 49.3 | 72.1 | <.001 |
| Previous ischemic heart disease, % | 21.2 | 13.4 | .095 |
| Interventional procedure | |||
| Crossover rate, % | 8.8 | 3.0 | .02 |
| Primary vascular access | <.001 | ||
| TRUA, % | 51.5 | 93.1 | |
| TFA, % | 44.0 | 5.6 | |
| GPIIb/IIIa inhibitors | 56.1 | 78.8 | .02 |
| Stent thrombosis, % | 7.3 | 3.6 | .04 |
| Needle-guidewire time, min | 23.1 (16.8) | 20.6 (12.4) | .142 |
| Contrast volume, mL | 167 (67) | 159 (56) | .319 |
| Fluoroscopy time, min | 19.2 (12.4) | 14.3 (8.9) | .001 |
| Successful primary angioplasty, % | 80.3 | 95.9 | .002 |
BMI, body mass index; BSA, body surface area; GPIIb/IIIa, glycoprotein IIb/IIIa; HT, hypertension; TFA, transfemoral vascular access; TRUA, transradial/ulnar access.
In the analysis of crossover rates according to the primary vascular access route, more crossovers were associated with TRUA in this subgroup than with TFA (17.1% vs 0%; P=.028). Nonetheless, the PPCI success rate (77.4% vs 83.3%; P=.871), needle-guidewire time (22.4 [13.6] vs 24.9 [21.4] min; P=.596), fluoroscopy time (17.4 [9.7] vs 20.9 [14.7] min; P=.364), and contrast volume (171 [68] vs 162 [67] mL; P=.596) showed no differences in relation to the access used.
DISCUSSIONThis study presents the results (without exclusions) of a PPCI program in 1029 patients, in whom more than 93% of PPCI procedures were carried out using TRUA. The angioplasty success rate was nearly 96%, and crossover was required in only 3.0%. These values remained stable during the period studied, and the procedure-related parameters showed a continuing improvement (shorter needle-guidewire time and smaller contrast volume). In addition, a subgroup of patients was identified (older women) in whom primary use of TFA was greater. Nonetheless, in this less favorable subgroup, the use of TRUA did not affect the success rate of angioplasty or reperfusion time, although we did observe a higher crossover rate (10.9% vs 2.6%; P=.006). The same occurred in patients in cardiogenic shock, in whom the use of TRUA was associated with a higher crossover rate, but the variables related to angiographic success were unaffected. These results indicate that experienced operators can identify and use TRUA even in less favorable subgroups without a negative impact on reperfusion time. Therefore, in general, TRUA could be the recommended primary approach in the scenario of sufficient expertise.
Transradial/Ulnar Access in Primary Angioplasty: Fewer Adverse EventsIn 2011, the RIVAL trial results were published, the largest clinical trial to date (more than 7000 patients) comparing TRUA with TFA in patients with an acute coronary syndrome. Adverse event rates were similar in the 2 groups, but there were fewer local vascular complications in patients with TRUA.13 Recently, the RIVAL results were reported for the subgroup of patients with ST-segment elevation acute coronary syndrome. Not only was there a reduction in the primary outcome, complications due to the vascular approach, with a benefit for radial access, but there was also a significant reduction in the composite outcome of death, infarction, stroke, and non-coronary artery bypass graft-related major bleeding (3.1% vs 5.2%; P=.026), and in all-cause death (1.3% vs 3.2%; P=.006).16 These results, which concur with those obtained in the RIFLE-STEACS,17 add a net clinical benefit to the results of most related observational studies and clinical trials published to date.5–11,18–25 Although the benefits with regard to bleeding seem to clearly favor radial access, the results of the recent STEMI-RADIAL clinical trial found no differences in major events or in 30-day mortality between the 2 access routes.26
Routine Use of the Transradial/Ulnar Approach: Crossover and Success of Primary AngioplastyAlthough the literature contains a great deal of data favoring TRUA, results from large clinical trials13,17 are needed to confirm the benefits of this technique. Because of this lack of definitive evidence, systematic use of TRUE in actual practice is not widespread, and there is little available real-life data on its applicability and reproducibility. In 2011, use of the radial approach in percutaneous coronary procedures in Spain exceeded the femoral approach for the first time (55.5% of all angioplasties).27
The crossover rates with this technique published to date have varied considerably, from 0% to more than 10%.5–7,9 In the RIVAL study, a 5.3% crossover rate was documented in PPCI,13 which fell to 4.4% in the tertile of centers with highest volume. In one of the few studies focussed on routine use of TRUA in PPCI, a group from The Netherlands reported a crossover rate of 3.8% that decreased with the learning curve to 1.5%.28 In contrast, the published success rates of PPCI with TRUA have been more stable and are not lower than those of TFA.5–7 In our series, the crossover rate was 3.0%, and the angioplasty success rate exceeded 95%. These parameters were unaffected by the greater complexity of patients who are currently treated by PPCI or the greater use of devices.
Although the need for crossover in our series was associated with longer fluoroscopy time, longer needle-guidewire time, a larger volume of contrast material, and lower PPCI success rates, we believe that these results were not solely due to the delay in obtaining a vascular access, but also to the possibility that the patients and procedures in the group requiring crossover may have been more complex. We consider that these results can be accepted in groups with low crossover rates.
Transradial/Ulnar Access in Less Favorable SubgroupsPrimary use of TFA in our series was associated with women older than 75 years. These 2 variables have been predictive of crossover in other series.28 The probable causes are a smaller diameter of the wrist arteries, which leads to atherosclerotic involvement and stenosis, increased vascular stiffness, and/or arterial loops. In our opinion, primary use of TFA in our center represents a “surrogate” indicator of the need for crossover. However, the use of TRUA in older women did not affect reperfusion time, although the crossover rate was greater.
Similar results were obtained in patients in cardiogenic shock; TRUA was used in more than half of these patients. Until the results of randomized studies become available and assuming a higher crossover rate in this subgroup, cardiogenic shock does not seem to be a contraindication for the use of TRUA, and furthermore, it frees both femoral arteries for potential implantation of devices for circulatory assistance.29
Study LimitationsSince this is a retrospective study in a patient series, the results may have been affected by unidentified confounding variables. Furthermore, the absence of an adequate control group may limit interpretation of the results. Lastly, extrapolation of our results to other centers should be done with caution. To obtain results similar to ours with TRUA, training and a sufficient volume of patients is required.
CONCLUSIONSTRUA can be safely and effectively used in most PPCI procedures with high success rates and little need for crossover. In certain subgroups with less favorable characteristics, such as older women and patients in cardiogenic shock, success rates and time to reperfusion are unaffected, but there is an increased need for crossover. Therefore, use of a femoral access should be considered early on in these patients.
TRUA should be the primary vascular access of choice in most patients undergoing PPCI.
CONFLICTS OF INTERESTNone declared.