Multiparametric scores have been designed for better risk stratification in Brugada syndrome (BrS). We aimed to validate 3 multiparametric approaches (the Delise score, Sieira score and the Shanghai BrS Score) in a cohort with Brugada syndrome and electrophysiological study (EPS).
MethodsWe included patients diagnosed with BrS and previous EPS between 1998 and 2019 in 23 hospitals. C-statistic analysis and Cox proportional hazard regression models were used.
ResultsA total of 831 patients were included (mean age, 42.8±13.1; 623 [75%] men; 386 [46.5%] had a type 1 electrocardiogram (ECG) pattern, 677 [81.5%] were asymptomatic, and 319 [38.4%] had an implantable cardioverter-defibrillator). During a follow-up of 10.2±4.7 years, 47 (5.7%) experienced a cardiovascular event. In the global cohort, a type 1 ECG and syncope were predictive of arrhythmic events. All risk scores were significantly associated with events. The discriminatory abilities of the 3 scores were modest (particularly when these scores were evaluated in asymptomatic patients). Evaluation of the Delise and Sieira scores with different numbers of extra stimuli (1 or 2 vs 3) did not substantially improve the event prediction c-index.
ConclusionsIn BrS, classic risk factors such as ECG pattern and previous syncope predict arrhythmic events. The predictive capabilities of the EPS are affected by the number of extra stimuli required to induce ventricular arrhythmias. Scores combining clinical risk factors with EPS help to identify the populations at highest risk, although their predictive abilities remain modest in the general BrS population and in asymptomatic patients.
Keywords
Several clinical variables have been demonstrated to predict a worse outcome in patients with Brugada syndrome (BrS).1–5 Prominent examples include the presence of previous syncope or cardiac arrest and a spontaneous type 1 electrocardiogram (ECG) pattern.3 The performance of an electrophysiological study (EPS) has been suggested as a valuable predictor of sudden cardiac death (SCD). Specifically, the number of extra stimuli that induce ventricular arrythmias (VA) is thought to be a relevant prognostic indicator.6 Unfortunately, at present, there is no consensus on the value of the EPS in predicting outcomes, nor is there agreement on the predictive value of family history of SCD. Hence, individual factors lack sufficient specificity to predict outcomes and guide therapy, including the need for an implantable cardioverter-defibrillator (ICD).
To overcome the limited value of these individual factors, several authors have advised the use of combined approaches that include clinical, electrocardiographic and, at times, electrophysiological parameters.7–10 In 2010, Delise et al.7 reported the usefulness of a score that includes both clinical and EPS parameters in individuals with BrS and no previous cardiac arrest. Similarly, Pedro Brugada's group advised a clinical score that incorporated ECG pattern, early familial SCD antecedents, inducible EPS, presentation as syncope or as aborted SCD and sinus node dysfunction, with high predictive performance.8 In 2018, the proposed Diagnostic Score System, referred to as the Shanghai BrS score, based on the available published reports and on weighted coefficients derived from limited datasets, including the presence of a spontaneous type 1 ECG, fever or drug-induced type 1 ECG, history of arrhythmia or arrhythmic syncope, family history, and the results of a genetic test, showed its value for the diagnosis and risk stratification of patients with BrS9 (table 1). However, these models have only been validated by Letsas et al.10 in a small series and more recently by Probst et al.11 Importantly, their usefulness according to the number of extra stimuli inducing VA is unknown. Finally, there is little available information on their predictive value in asymptomatic patients.
Individual prognostic variables in the 3 risk scores under study
| Score | Variables | Categories |
|---|---|---|
| Delise et al.,7 | 1) Family history of SCD2) Syncope3) Spontaneous type 1 ECG4) Positive EPS | Individuals at higher risk are those with a baseline type 1 ECG pattern who have at least 2 of the following risk factors:- Syncope- Family history of SCD- Positive EPS |
| Sieira et al.8 | 1) Syncope (2 points)2) Aborted SCD (4 points)3) Spontaneous type 1 ECG (1 point)4) Sinus node dysfunction (3 points)5) Early familial antecedents of SCD in first-degree relatives (1 point)6) Inducible VA (2 points) | A score >2 showed a significantly higher event probability |
| Shanghai BrS Score9 | I. ECG (12-lead/ambulatory)- Spontaneous type 1 ECG at nominal or high leads (3.5 points)- Fever-induced type 1 ECG at nominal or high leads (3 points)- Type 2 or 3 ECG pattern that converts with drug provocation challenge (2 points)II. Clinical history- Unexplained cardiac arrest or documented VF/polymorphic VT (3 points)- Nocturnal agonal respirations (2 points)- Suspected arrhythmic syncope (2 points)- Syncope of unclear mechanism/unclear etiology (1 point)- Atrial flutter/fibrillation in patients <30 y without alternative etiology (0.5 points)III. Family history- First- or second-degree relative with definite BrS (2 points)- Suspicious SCD (fever, nocturnal, Brugada aggravating drugs) in a first- or second-degree relative (1 point)- Unexplained SCD at <45 y in first- or second-degree relative with negative autopsy (0.5 points)IV. Genetic test result- Probable pathogenic mutation in BrS susceptibility gene (0.5 points) | - Moderate risk: 3.5- High risk: 4 to 5- Highest risk ≥ 5.5 |
BrS, Brugada syndrome; ECG, electrocardiogram; EPS, electrophysiological study; SCD, sudden cardiac death; VA, ventricular arrythmia; VF, ventricular fibrillation; VT, ventricular tachycardia.
We aimed to validate these multiparametric approaches in a wide cohort of patients with BrS and EPS by determining their predictive abilities via the number of extra stimuli required to induce VA, as well as the presence of previous symptoms. A secondary endpoint was to analyze predictors of a positive EPS.
METHODSStudy inclusionThis is a retrospective registry of patients diagnosed with BrS and with previous EPS between 1998 and November 2020 in 1 Portuguese and 23 tertiary Spanish hospitals. The registry was conducted according to the Declaration of Helsinki and was approved by the institutional review board of each center. The participating centers included consecutive patients with BrS and EPS. BrS was diagnosed after an episode of aborted SCD, during syncope workup, in asymptomatic patients with a suggestive ECG pattern during routine examination, or as a consequence of familial screening after diagnosis of BrS in a family member.
Syncope was defined as loss of consciousness in accordance with the current literature consensus.12–15 For the purpose of this study, attempts were made to differentiate vasovagal from arrhythmic causes and only episodes of syncope of cardiovascular origin were included. Patients were included if they satisfied the screening criteria above and had a type 1 Brugada pattern on ECG at baseline on at least 1 occasion or after provocation with a class I antiarrhythmic drug (depending on availability in the participating hospitals, either ajmaline or flecainide/procainamide were used). A type 1 ECG pattern was defined as a prominent coved ST-segment elevation displaying J-wave amplitude or ST-segment elevation ≥ 0.2mV at its peak followed by a negative T-wave.14
The primary outcome included SCD or high-voltage defibrillator therapy (ie, shocks) for polymorphic ventricular tachycardia or ventricular fibrillation (VF), as determined through defibrillator interrogations during follow-up. We classified only defibrillator shocks for polymorphic ventricular tachycardia or fibrillation; VA terminated by antitachycardia pacing were not classified as meeting the primary outcome.
Follow-up protocolIn the absence of symptoms or device therapy, patients were seen routinely every 6 to 12 months for clinical review and device interrogation, in accordance with the local practice protocol. ICD programming was left to the discretion of the referring electrophysiologist. However, following the 2006 recommendations, programming of a single VF zone above 210 to 220 bpm was advocated. Those patients who were not followed up in the original institution were contacted through telephone interview.
Electrophysiologic studyProgrammed ventricular stimulation was consistently performed at the apex of the right ventricle (RV) and in some centers, also from the right ventricular outflow tract. Stimulation included 2 to 3 pacing cycle lengths (600, 500, and 430 ms) with up to 3 extra stimuli. The extra stimuli were anticipated in 10 ms decrements up to a shortest coupling interval of 200 ms. A positive EPS result was defined as the induction of a sustained VA (> 30seconds) or one that required shock. A negative EPS outcome was attributed when no VA or a short-lived, self-terminating VA was induced but did not require shock.
Statistical analysisStatistical analyses were performed with SPSS software version 21 (SPSS Inc, United States) and R version 3.6 (R Foundation for Statistical Computing, Austria).16 Continuous variables are reported as mean±standard deviation and categorical variables as frequencies and percentages. A comparison of baseline characteristics between groups was performed using the chi-square test for categorical variables and the Student t test/Mann-Whitney U test to compare ranks of continuous variables. The c-index was used to assess the discriminatory capability of the model (concordance).
Cumulative incidence curves were plotted to assess the survival of patients according to their risk of competing events. Time 0 corresponds to the moment of the EPS. A competing risk is an event whose occurrence either precludes the occurrence of another event under examination or fundamentally alters the probability of the occurrence of this other event.
Fine and Gray competing risk regression models were used to predict the individual's overall risk (incidence) of an event. Risk was quantified as a Fine-Gray subdistributional hazard ratios (SHR), with 95% confidence intervals (95%CI). The reference category in each of the 4 scores corresponded to that with the lowest score (0 for the Delise score,7 <2 for the Sieira score,8 and ≤ 3 points for the Shanghai BrS score).9 For each cutpoint of the scores and EPS, the time-dependent positive predictive value (PPV) and negative predictive value (NPV) were estimated by inverse probability of censoring weighting, considering competing risks.
RESULTSClinical characteristics and indication for implantable cardioverter-defibrillator implantationPatient characteristicsA total of 831 patients were available for the analysis. The baseline characteristics of the patients in the final database are summarized in table 2. Mean follow-up was 10.18±4.77 years.
Demographic characteristics of the study population
| Total population (N=831) | |
|---|---|
| Age diagnosis, y | 42.8±13.1 |
| Male/female | 561 (77)/208 (25) |
| Spontaneous type 1 | 386 (46.5) |
| Drug-induced type 1 | 541 (65.1) |
| Ajmaline/flecainide | 156 (18.8)/398 (47.9) |
| Fever-induced type 1 | 30 (3.6) |
| Family history of SCD | 282 (33.9) |
| Asymptomatic | 677 (81.5) |
| Syncope | 127 (15.3) |
| Previous history of SCD | 28 (3.4) |
| Atrial fibrillation | 42 (5.1) |
| Genetic positive | 126 (15.2) (n=390) |
| Positive EPS (3 extra stimuli) | 272 (32.7) |
| Positive EPS (2 extra stimuli) | 111 (13.4) |
| Drug-induced and positive EPS | 99 (11.9) |
| ICD implantation | 319 (38.4) |
| Mean Delise score | 1.3±1 |
| Mean Sieira score | 1.6±1.6 |
| Mean Shanghai BrS score | 3.8±1.3 |
| Sieira score | |
| ≤ 2 | 577 (69.4) |
| 3-5 | 238 (28.6) |
| 6-8 | 12 (1.4) |
| Delise score | |
| 1 | 305 (36.7) |
| 2 | 222 (26.7) |
| 3 | 88 (10.6) |
| 4 | 9 (1.1) |
| Shanghai score | |
| ≤ 3 | 103 (12.4) |
| 3.5 | 90 (10.8) |
| 4-5 | 104 (12.5) |
| ≥ 5.5 | 59 (7.1) |
BrS, Brugada syndrome; EPS, electrophysiological study; ICD, implantable cardioverter-defibrillator; SCD, sudden cardiac death.
Data are expressed as No. (%) or mean±standard deviation.
Ventricular arrhythmias (ventricular tachycardia/VF) were induced by means of programmed ventricular stimulation in 272 patients (32.7%). Specifically, VAs were induced with 1 or 2 extra stimuli in 111 patients (13.4%). In 4 patients (0.48%), information on the number of extra stimuli was missing.
The EPS was performed from the RV apex in 523 patients (62.9%) and from the RV apex and RV outflow tract in 245 (29.5%). In 63 patients, this information could not be recovered. Event rate varied between the 1-site (RV apex) vs 2-site protocol (RV outflow tract) (7.5% vs 2.4% respectively; P=.043). However, it did not show a statistical relationship in VA occurrence in the univariable Cox regression analysis of time-to-event.
On multivariable analysis, age at diagnosis (1.02 (1.01-1.04)), and type 1 ECG pattern (2.14, 1.38-3.33) and family history of SCD or previous syncope episodes were predictors of positive EPS (1,2 or 3 extra stimuli), with female sex (0.41, 0.27-0.62) being inversely associated (table 3). When the EPS was considered positive when VA were induced with 1 to 2 extra stimuli, previous syncope episodes were not predictors of positive EPS (table 3).
Logistic regression analysis for predicting the risk of positive electrophysiological study with 3 extra stimuli and with 1 to 2 extra stimuli
| Positive EPS (3 extra stimuli) | Positive EPS (1-2 extra stimuli) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariableodds ratio | P | Multivariableodds ratio | P | Univariableodds ratio | P | Multivariableodds ratio | P | |
| AUC | 0.79 | 0.82 | ||||||
| Intercept | 0.02 (0-0.08) | <.0001 | 0.0009 (0-0.01) | <.0001 | ||||
| Age diagnosis (years) | 1.02 (1.01-1.03) | .0003 | 1.05 (1.02-1.08) | .004 | 1.03 (1.01-1.04) | .0013 | 1.09 (1.05-1.14) | <.0001 |
| Female vs male | 0.39 (0.26-0.56) | <.0001 | 0.23 (0.08-0.58) | .003 | 0.39 (0.21-0.68) | .002 | 0.2 (0.05-0.63) | .012 |
| Spontaneous type 1 | 2.91 (2.16-3.94) | <.0001 | 3.74 (1.87-7.79) | .0003 | 3.19 (2.08-4.97) | <.0001 | 4.18 (1.71-11.15) | .0025 |
| Drug-induced type 1 | 0.4 (0.3-0.55) | <.0001 | 0.38 (0.25-0.57) | <.0001 | ||||
| Fever-induced Type 1 | 1.84 (0.87-3.84) | .1 | 2.03 (0.79-4.62) | .11 | ||||
| Family history of SCD | 1.18 (0.87-1.59) | .3 | 2.64 (1.3-5.45) | .008 | 1.18 (0.77-1.77) | .44 | 2.72 (1.14-6.62) | .024 |
| Syncope | 1.49 (1.01-2.2) | .04 | 2.64 (1.19-5.86) | .016 | 1.38 (0.81-2.29) | .22 | 1.81 (0.67-4.71) | .23 |
| Sudden cardiac death | 1.57 (0.71-3.34) | .25 | 1.8 (0.65-4.29) | .21 | ||||
| Genotype results, positive | 0.76 (0.41-1.37) | .36 | 0.9 (0.43-1.87) | .78 | 0.78 (0.36-1.62) | .51 | 0.94 (0.36-2.36) | .9 |
| Atrial fibrillation | 1.58 (0.83-2.95) | .15 | 1.11 (0.41-2.53) | .82 | ||||
| Hosmer-Lemeshow P value | .92* | .437* | ||||||
AUC, area under the curve; EPS, positive electrophysiological study; SCD, sudden cardiac death.
The numbers of patients belonging to each category are presented in table 4. During follow-up, 47 (5.7%) experienced an SCD or high-voltage defibrillator therapy (39 an appropriate ICD intervention and 8 patients sustained ventricular tachycardia, 2 of them were successfully rescued and 6 patients died) (figure 1). Fourteen patients died due to non-SCD/ICD shock.
Number of patients in each category in the present study and number of events in each category
| Score | Patients in each category | Events in each category | Asymptomatic patients in each category | Events in each category (asymptomatic patients) |
|---|---|---|---|---|
| Delise et al.7 | 0=187 (23)1=305 (37)2=222 (27)3=88 (11)4=9 (1) | 0=2 (4)1=4 (9)2=19 (40)3=9 (19)4=3 (6) | 0=184 (27)1=272 (40)2=176 (26)3=45 (7)4=0 (0) | 0=2 (10)1=4 (19)2=12 (57)3=3 (14)4=0 (0) |
| Sieira et al.8 | ≤ 2=577 (69)3-5=238 (29)6-8=12 (1) | ≤ 2=12 (26)3-5=30 (64)6-8=5 (11) | ≤ 2=530 (78)3-5=145 (21)6-8=0 (0) | ≤ 2=10 (48)3-5=11 (52)6-8=0 (0) |
| Shanghai BrS score9 | ≤ 3=103 (12)3.5=90 (11)4-5=104 (13)≥ 5.5=59 (7) | ≤ 3=2 (4)3.5=6 (13)4-5=7 (15)≥ 5.5=14 (30) | ≤ 3=103 (15)3.5=90 (13)4-5=78 (12)≥ 5.5=14 (2) | ≤ 3=2 (10)3.5=6 (29)4-5=4 (19)≥ 5.5=0 (0) |
BrS, Brugada syndrome.
The data are expressed as No. (%).
Schematic representation of the study population with the more relevant baseline characteristic and event rate during the follow-up period. CSH, cause-specific hazard; ECG, electrocardiogram; EPS, electrophysiological study; ICD, implantable cardioverter-defibrillator; PVC, premature ventricular complex; SCD, sudden cardiac death.
The median time for the occurrence of cardiac events after the EPS was 5.21±4.23 years (interquartile range, 1.3-6.8) and the median age when the malignant event occurred was 51.06±15.47 years. Among patients who sustained an event, 34 (72.3%) had a type 1 ECG pattern, 21 (44.7%) were previously asymptomatic, 21 (44.7%) had a family history of SCD (4 of them with age of the relative <35 years), 27 (57.4%) had a positive EPS (15 [31.9%] positive with 2 extra stimuli) and 10 (21.3%) had a previous history of SCD. The number of events in the total population and in those who were asymptomatic according to their baseline risk score is also presented in table 4.
The PPV and NPV were reported for the 3 scores, adjusted for competing risks (). As shown in a graphic approach, the PPV and NPV curves quantify the predictive values of the assessed scores, measured on a continuous scale with a censored failure time outcome.
Time-to-event, multivariable regression analysis, and c-indexWhen competing risks and time-to-event regression analyses were performed in the total cohort, a type 1 ECG pattern, a positive EPS with 1 or 2 extra stimuli and previous syncope were associated with arrhythmic events (table 2). In asymptomatic patients, a type 1 ECG pattern and a positive EPS with 1 or 2 extra stimuli were associated with arrhythmic events.
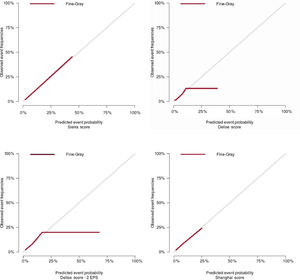
Stepwise increase in the risk of deathAll risk scores showed a trend toward an increase in the risk of events corresponding to the higher point ranges of the 3 scores under study (figure 2, table 4). The discriminatory and calibration ability of the 3 scores are represented in table 4 and figure 3. The c-indices reported in figure 2 are from Fine-Gray models for the global population and in asymptomatic patients. The evaluation of the models incorporating EPS (Delise and Sieira scores) with the number of extra stimuli (1 or 2 vs 3) did not significantly modify the 95%CI boundaries or substantially change the event prediction c-index.
The present study sought to evaluate the role of 3 multiparametric risk scores in patients with BrS who underwent an EPS. Our main findings were: a) in accordance with existing data, classic risk factors such as ECG pattern and previous syncope predict future arrhythmic events; b) the number of extra stimuli required to induce VA (1 or 2 vs 3) has a significant impact on the predictive capabilities of the EPS; c) scores that combine clinical risk factors with EPS help to identify the populations at highest risk, although their predictive capacities remain modest in the general population and in asymptomatic patients.
Individual risk factorsTo date, the factor with the highest predictive value remains the baseline ECG pattern, often useful as the basis for decision-making.12,13 Nevertheless, it is important to highlight that one third of patients with events did not have a baseline type 1 ECG pattern, highlighting the dynamic nature of BrS. Besides the ECG, a history of arrhythmic events is considered a harbinger for recurrence.12–14,17 In our sample, the presence of previous syncope was also a robust predictive factor of future events. On the other hand, the role of a family history of SCD in predicting arrhythmic events remains controversial.7,12,17–20. In the study by Delise et al.,7 the presence of family history of SCD did not independently correlate with increased arrhythmic risk. Of note, the characterization of family history showed slight differences according to the score (“early SCD in first-degree relatives” in the Sieiras score; “before the age of 40” in the Delise score, and “family history in first- or second-degree relatives” (with different weights according to definitive diagnosis BrS [2 points], suspicious SCD [1 point] related to BrS or unexplained SCD at <45 years of age5 in the Shanghai BrS score). A recent study established a genetic predictive score based on the presence of the SCN5A mutation and polymorphism in other genes associated with arrhythmic risk.20 It remains to be seen whether the incorporation of both family history and genetic predictors could better identify those at high genetic risk of SCD. In our sample, neither the presence of family history nor a perceptible genetic mutation (performed in more than 300 patients) were reliable predictors for further events. The issue of the usefulness of EPS has been addressed by several consensus documents and multiple relevant meta-analyses. Unfortunately, conflicting evidence still exists regarding its prognostic value and, at present, it remains a IIb indication.21,22 However, it has gained value in the last few years with the publication of meta-analyses.5 Interestingly and in agreement with our own and previous data, induction with fewer extra stimuli was associated with higher risk. Nonetheless, and as already observed by Sroubek et al.,4 a negative EPS does not portent a low VA risk, particularly in patients with high-risk clinical features. Our data are even more disconcerting. We followed up 20 patients with a negative EPS (32 if the EPS is considered positive when VA were induced with 1-2 extra stimuli) and who developed cardiac events in the long term. Also relevant is the fact that its NPV and PPV are dependent on the follow-up period (). Although this observation has never been analyzed, in our opinion, its predictive abilities seem to be time-dependent, and it could be argued that EPS should be re-evaluated at certain time intervals.
Combined risk factorsTo overcome the limited value of the aforementioned individual factors, several authors advised the use of combined approaches. This is employed in some recommendations, for example, the Japanese Cardiology Society recommended ICD implantation for primary prevention in patients with BrS according to the number of risk factors (syncope, positive EPS, and family history of SCD).23 A potential advantage of the multiparametric approach lies in the enhanced specificity of combined risk factors compared with their individual components. One essential benefit of combined risk stratification is that patients without any risk factors have a sufficiently low incidence of arrhythmic events to justifiably circumvent specific therapy.3 In their multicenter studies (including 111 individuals, with EPS in 59), Letsas et al.10 evaluated the predictive ability of these risk scores. The Kaplan-Meier analysis showed that individuals displaying at least 4 risk factors (ECG, syncope, family history, EPS, fragmented QRS, and prolonged QRS in lead V2) were at increased risk of developing arrhythmic events. The ECG pattern, the presence of syncope and a positive EPS (albeit the Shanghai BrS score, which does not evaluate the role of the EPS) are common risk factors in the 3 models evaluated. Interestingly, the presence of a family history of SCD—although not statistically significant as an isolated variable at least in the Delise score—seems to help calibrate the final model and, although it was not the case in our cohort, it might be of help in borderline cases. More recently, Probst et al.11 evaluated the accuracy of the Shanghai BrS and Siera score. Predictive capacity was estimated by an area under the curve of 0.73 (0.67-0.79) and 0.71 (0.61-0.81). Of note, and in line with our results, risk scores did not allow stratification of the risk of arrhythmic events in intermediate-risk patients.
In our cohort, the 3 scores and a positive EPS with either 1 to 2 or 3 stimuli exhibited a low PPV and high mean NPV (taking into account competing risks). However, NPV exhibited a strong temporal dependence, being high (up to 100% at the beginning of follow-up, when no events were recorded) and with a steep decline at longer follow-up times (). The FINGER trial reported that patients without symptoms and without a spontaneous type 1 ECG pattern were found to have a very low risk of SCD regardless of VF inducibility during EPS.11 In our sample, although the number of events in patients with few risk factors was low (table 2), it was not negligible. Two events occurred in patients with 0 risk factors in the Delise7 and Shanghai9 BrS scores, and events occurred in 12 patients with <2 points in the Sieira score.8 Likely, the inclusion of a significantly higher number of asymptomatic patients in the present study than in these series (154,7 74,10 269,8 and 2719 vs 627 in the present study) could have attenuated the predictive value of the scores. The addition of atrial fibrillation or sinus node dysfunction to the models (as proposed by the Shanghai9 and Sieria score,8 respectively) may have improved their predictive abilities, although the latter did not result in a significant change in our sample, most likely due to the low number of patients with these diagnoses. Indeed, it seems to be an uncommon diagnosis among patients with BrS, not only in our series. In Sieira series, only 2 out of 269 asymptomatic patients had this diagnosis.8
To sum up, we believe that these data emphasize the urgent need for better categorization tools in low-risk patients than the current conventional risk factors. Until such tools are available, particularly in the subset of asymptomatic BrS patients, and particularly in those with a spontaneous type 1 ECG pattern, we suggest the use of scores that incorporate the ECG and the EPS. However, as previously mentioned, due the lack of specificity of the EPS, it can lead to an overtreatment of patients at low risk. We believe that the management of these patients should be individualized and complemented with other advised markers such as the presence of fragmented QRS complexes, early repolarization, signal-averaged techniques, microscopic T-wave alternans,3 ventricular refractory period <200 ms,13 along with clinical factors such as atrial fibrillation and sinus node dysfunction.8,9 In contrast, a negative EPS should not be linked to low VA risk and these patients should also undergo periodic evaluation. Moreover, although we hypothesized that the adjustment of these risk scores by incorporating the number of extra stimuli required to induce VA could improve their predictive abilities, this was not the case (table 5), and induction of VA with 1 or 2 extra stimuli (compared with 3) did not improve the predictive performance measured by concordance index. Nevertheless, due to the positive value of the EPS with 1 or 2 extra stimuli at 1 right ventricular site compared with 3 extra stimuli in our model (together with age at diagnosis and type 1 ECG pattern) this protocol might be encouraged instead of the traditional one using 3 extra beats at 2 right ventricular sites.
Coefficients and c-indices of models that predict events in the global population and in asymptomatic patients, according to the number of extra stimuli that induced ventricular arrhythmias (1 or 2 vs 3). Adjusted by competing risk of a person dying without experiencing the event of interest
| CIF event | ||||
|---|---|---|---|---|
| c-index | SHR | 95%CI | P | |
| Model in the global population | ||||
| Sieira | 0.81 | 1.78 | 1.53-2.07 | <.0001 |
| Sieira with 1-2 EPS | 0.80 | 1.83 | 1.56-2.13 | <.0001 |
| Delise score* | 0.77 | 2.40 | 1.75-3.28 | <.0001 |
| Delise with 1-2 EPS* | 0.76 | 2.71 | 1.91-3.85 | <.0001 |
| Shanghai score | 0.80 | 1.75 | 1.38-2.22 | <.0001 |
| Model in asymptomatic persons | ||||
| Sieira | 0.69 | 1.70 | 1.28-2.27 | .0001-.001 |
| Sieira with 1-2 EPS | 0.66 | 1.79 | 1.32-2.44 | .0001-.001 |
| Delise score | 0.72 | 2.09 | 1.36-3.21 | .0001-.001 |
| Delise with 1-2 EPS | 0.69 | 2.31 | 1.42-3.76 | .0001-.001 |
| Shanghai score | 0.64 | 1.15 | 0.78-1.69 | |
95%CI, 95% confidence interval; CIF, cumulative incidence Fine-Gray model; EPS, electrophysiological study; SHR, subdistributional hazard ratio (exponentiated).
Patients with Brugada syndrome and a score rate between 3 and 5 or between 6 and 8 in the Sieira score or individuals at higher risk according to the Delise score (type 1 ECG pattern and at least 2 of the following risk factors, syncope, family history of SCD or a positive EPS) (see table 1) but in whom the EPS was considered positive when inducible ventricular arrhythmias were provoked with 1 to 2 extra stimuli (if ventricular arrhythmias were induced with 3 extra stimuli, the EPS was categorized as negative).
Although the clinical profile of syncopal episodes was taken into account to exclude patients with noncardiogenic syncope, it is plausible that some vasovagal syncopal episodes could have been included, which could have swayed study outcomes. This study used a national registry, which may be subject to a referral bias and regional characteristics that vary among populations. Nevertheless, we believe that it is a good indicator of nationwide practice and outcomes. Moreover, the cohort included only BrS patients who underwent EPS. The referral for EPS was left to physician discretion rather than standardized indications and may have varied between centers. Therefore, the study results could not be uniformly extrapolated to the general BrS population. Arrhythmic events were classified according to SCD and ICD interrogation. Nonetheless, because ventricular tachyarrhythmias may terminate spontaneously, appropriate ICD shock is not synonymous with SCD. Moreover, not all SCDs can be attributable to BrS. This nuance could have led to an overestimation of the number of events.
An important limitation of the present study is that the risk scores could not be calculated in the entire study population due to missing information. This was particularly true of the Shanghai score (calculated in only 415 out of 831 individuals). As such, this study did not aim to compare the scores with each other, but instead to express their predictive abilities in our patients with BrS. We performed a sensitivity analysis removing all rows with missing values in the Shanghai score, without changes in either the interpretation of multivariable models or event prediction for the cohort. However, in asymptomatic patients, the loss of power due to removal of missing data caused the Delise score to be nonpredictive and a lack of convergence in the Shanghai score.
In a disease with a life-long risk of lethal arrhythmias, although comparatively longer than the vast majority of series, the present study might not have sufficient power to definitely exclude or confirm the predictive value of any of the aforementioned parameters. Moreover, the rate of cardiac events could be too small to allow firm conclusions to be drawn; further studies with a larger number of patients will be needed to confirm the conclusions of this study.
Finally, only the Shanghai BrS and Sieira scores seem to be adequately calibrated, whereas the Delise score showed poor calibration. The results of our study should be considered in light of this limitation.
CONCLUSIONSIn patients with BrS, classic risk factors such as ECG pattern and previous syncope predict future arrhythmic events. The number of extra stimuli required to induce VAs (1 or 2 vs 3) substantially impacts the predictive capabilities of the EPS. Finally, scores that combine clinical risk factors with EPS help to identify the populations at highest risk, although their predictive capacities remain modest in the general population and in asymptomatic patients.
FUNDINGM. Rodríguez-Mañero holds a grant from the “Fundación Mutua Madrileña” for the study of Brugada syndrome. E. Arbelo, and J. Brugada have received support for this study by Instituto de Salud Carlos III (FIS PI16/01203) co-funded by ERDF/ESF, “Investing in Your Future” and Fundació La Marató de TV3 (Projecte 245/U/2020).
AUTHORS’ CONTRIBUTIONSM. Rodríguez-Mañero and A. Baluja conceived the study. Both authors contributed equally to the drafting of this manuscript. All authors developed the hypothesis, supervised the findings of this work, discussed the results, and contributed to the final manuscript.
CONFLICTS OF INTERESTM.Á. Arias is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. The other authors declare no conflicts of interest.
- -
In Brugada syndrome, individual risk factors are insufficiently specific to predict outcomes and guide therapy.
- -
To overcome the limited value of these individual factors, it has been advised to use combined approaches that include clinical, electrocardiographic, and electrophysiological parameters.
- -
These models have only been validated in small series. Importantly, their usefulness according to the number of extra stimuli inducing VA is unknown.
- -
We aimed to validate 3 multiparametric approaches in a cohort of patients with Brugada syndrome and electrophysiological study.
- -
All risk scores were significantly associated with events. However, the discriminatory abilities of the 3 scores were modest, particularly when these scores were evaluated in asymptomatic patients. Evaluation of the scores with different numbers of extra stimuli (1 or 2 vs 3) did not substantially improve the event prediction c-index.
- -
These data emphasize the urgent need for better categorization tools than current conventional risk factors in low-risk patients.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.07.007