Atrial fibrillation (AF) is a complex medical condition with poorly understood mechanisms.1,2 In some cases, it leads to heart failure (HF), increasing mortality. AF can also cause HF without underlying cardiac issues, known as arrhythmia-induced cardiomyopathy (AiCM).3 This form of HF can be improved with rate or rhythm control.4 Diagnosing AiCM is currently impossible without follow-up documentation of left ventricular ejection fraction (LVEF).
Recently, the ANTWOORD study5 introduced a new prediction model, the Antwerp score, to identify patients with systolic HF due to AF whose LVEF improved after rhythm control via catheter ablation. We aimed to validate the Antwerp score in a retrospective analysis of a prospectively enrolled cohort (SWISS-AF-PVI, NCT03718364) of AF patients undergoing pulmonary vein isolation.6
The predictive ability of the Antwerp score was assessed using receiver operating characteristics (ROC) curves and calibration plots. Continuous variables were compared using the Mann-Whitney U test or the t-test. Low percentages (indexed left atrial volume [LAVI] 7.2%) of missing values were statistically imputed. All statistical analyses were performed using R version 4.2.1.
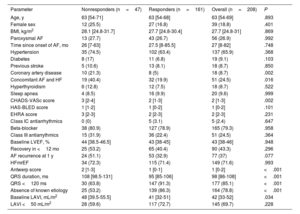
Between May 2010 and January 2022, 1665 patients underwent catheter ablation for AF. Of these, 1447 (87%) were excluded due to baseline LVEF ≥ 50% and 10 (0.6%) were lost to follow-up, leaving a total of 208 patients (median age, 63 [54-69] years, 19% women) in the final analysis; LVEF was 40% to 50% in 72% and was <40% in 28% (median LVEF, 43% [38-46], table 1). In this cohort, 161 patients (77%) were responders and 47 (23%) were nonresponders. The median length of follow-up was 30 [20-34] months. Coronary artery disease was significantly less frequent in responders than in nonresponders (5% vs 23%; P=.002) as was concomitant diagnosis of AF and HF (20% vs 40%; P=.016). Responders also had significantly shorter QRS duration than nonresponders (95 [85-106] vs 108 [99-131]; P <.001), smaller LAVI (41 [32-51] vs 48 [39.5-55.5]; P=.03) and more frequently had HF without known etiology (86% vs 53%; P <.001). The type of AF was comparable between responders and nonresponders (paroxysmal AF 27% vs 28%; P=.99). AiCM was clinically suspected by the treating physician in 34 patients (17% responders vs 13% nonresponders; P=.61). The median time to LVEF recovery in responders was 8 [3-22] months. Of these, 20 patients showed LVEF recovery after more than 1 year, despite not experiencing AF recurrence: 75% had hypertensive heart disease, 15% obstructive sleep apnea, and 10% diabetes.
Baseline characteristics
| Parameter | Nonresponders (n=47) | Responders (n=161) | Overall (n=208) | P |
|---|---|---|---|---|
| Age, y | 63 [54-71] | 63 [54-68] | 63 [54-69] | .893 |
| Female sex | 12 (25.5) | 27 (16.8) | 39 (18.8) | .401 |
| BMI, kg/m2 | 28.1 [24.8-31.7] | 27.7 [24.8-30.4] | 27.7 [24.8-31] | .869 |
| Paroxysmal AF | 13 (27.7) | 43 (26.7) | 56 (26.9) | .992 |
| Time since onset of AF, mo | 26 [7-63] | 27.5 [8-85.5] | 27 [8-82] | .748 |
| Hypertension | 35 (74.5) | 102 (63.4) | 137 (65.9) | .368 |
| Diabetes | 8 (17) | 11 (6.8) | 19 (9.1) | .103 |
| Previous stroke | 5 (10.6) | 13 (8.1) | 18 (8.7) | .850 |
| Coronary artery disease | 10 (21.3) | 8 (5) | 18 (8.7) | .002 |
| Concomitant AF and HF | 19 (40.4) | 32 (19.9) | 51 (24.5) | .016 |
| Hyperthyroidism | 6 (12.8) | 12 (7.5) | 18 (8.7) | .522 |
| Sleep apnea | 4 (8.5) | 16 (9.9) | 20 (9.6) | .999 |
| CHADS-VASc score | 3 [2-4] | 2 [1-3] | 2 [1-3] | .002 |
| HAS-BLED score | 1 [1-2] | 1 [0-2] | 1 [0-2] | .101 |
| EHRA score | 3 [2-3] | 2 [2-3] | 2 [2-3] | .231 |
| Class IC antiarrhythmics | 0 (0) | 5 (3.1) | 5 (2.4) | .647 |
| Beta-blocker | 38 (80.9) | 127 (78.9) | 165 (79.3) | .958 |
| Class III antiarrythmics | 15 (31.9) | 36 (22.4) | 51 (24.5) | .364 |
| Baseline LVEF, % | 44 [38.5-46.5] | 43 [38-45] | 43 [38-46] | .948 |
| Recovery in <12 mo | 25 (53.2) | 65 (40.4) | 90 (43.3) | .296 |
| AF recurrence at 1 y | 24 (51.1) | 53 (32.9) | 77 (37) | .077 |
| HFmrEF | 34 (72.3) | 115 (71.4) | 149 (71.6) | .993 |
| Antwerp score | 2 [1-3] | 1 [0-1] | 1 [0-2] | <.001 |
| QRS duration, ms | 108 [98.5-131] | 95 [85-106] | 98 [86-108] | <.001 |
| QRS <120 ms | 30 (63.8) | 147 (91.3) | 177 (85.1) | <.001 |
| Absence of known etiology | 25 (53.2) | 139 (86.3) | 164 (78.8) | <.001 |
| Baseline LAVI, mL/m2 | 48 [39.5-55.5] | 41 [32-51] | 42 [33-52] | .034 |
| LAVI <50 mL/m2 | 28 (59.6) | 117 (72.7) | 145 (69.7) | .228 |
AF, atrial fibrillation; BMI, body mass index; EHRA, European Heart Rhythm Association; LAVI, indexed left atrial volume; LVEF, left ventricular ejection fraction; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction.
The data are expressed as median [interquartile range] or No. (%).
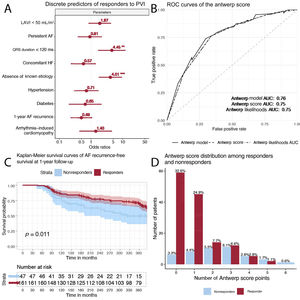
On univariable analysis, the factors significantly associated with lack of LVEF recovery after pulmonary vein isolation were QRS duration as a continuous variable (P <.001), LAVI (P=.005), concomitant AF and HF (P=.003), and absence of known etiology of HF (P <.001). These findings were mirrored when using the binary versions of the variables apart from LAVI <50mL/m2 (P=.08). On multivariable analysis, after adjustment for the differences in baseline parameters between the cohorts, only QRS duration <120ms (OR, 4.41; 95%CI, 1.68-11.88; P=.002) and the absence of known HF etiology (OR, 4.10; 95%CI, 1.80-9.42; P <.001) remained significantly associated with lack of LVEF recovery (figure 1).
A: forest plot showing the odds ratios of various discrete parameters in the prediction of LVEF recovery after pulmonary vein isolation. B: receiver operating characteristic curves of the Antwerp prediction model (thick continuous line), Antwerp score (points ranging from 0-6, dashed line), and likelihoods provided in the Antwoord study (dotted line). C: atrial fibrillation recurrence-free survival of responders vs nonresponders. D: distribution of Antwerp points between responders and nonresponders. AF, atrial fibrillation; AUC, area under the curve; HF, heart failure; LAVI, indexed left atrial volume.
When ROC analysis was used, the Antwerp score as a prediction model had an area under the curve (AUC) of 0.76 (0.68-0.84), while the score itself (between 0 and 6, depending on the number of positive criteria in each patient) had an AUC of 0.75 (95%CI, 0.67-0.83), and the likelihoods provided in the ANTWOORD study had an AUC of 0.75 (95%CI, 0.67-0.83). Calibration plots showed slopes between 0.39 and 0.69. A similar AUC (0.76) was obtained when we evaluated a nonimputed dataset. The score performed similarly in patients without AF recurrence at 12 months (AUC: 0.79). The likelihood ratio of a model containing QRS <120ms and the absence of known etiology performed similarly to the complete Antwerp model (Pchisq=.77).
This secondary analysis of a large prospective, multicenter study was performed to externally validate the Antwerp score and examine its potential clinical generalizability. The Antwerp score was previously found to accurately predict LVEF recovery after AF ablation, and in this slightly healthier validation cohort, it demonstrated a modest predictive power for LVEF recovery. QRS duration and the absence of a known etiology of HF were confirmed as excellent predictors of LVEF recovery. The score performed better in predicting response in patients with a low probability of LVEF recovery (5 or 6 points), while its performance in patients with a high probability of LVEF recovery (0, 1 or 2 points) was poor. Only 17% of responders had a prior clinical diagnosis of AiCM, which was not a significant factor in determining recovery after catheter ablation, indicating the limited possibility of a diagnosis of this entity prior to treatment. Consecutive redo procedures and/or adjuvant antiarrhythmic drug therapy might be required during follow-up to ensure LVEF recovery. LVEF might recover in some patients after more than 1 year if there is underlying heart disease and/or comorbidities even without AF recurrence. A score such as the Antwerp score5 is a necessity and further studies to identify other predictive parameters are required.3
Several differences between the derivation and validation cohorts should be noted and might have affected the performance of the Antwerp score: the proportion of responders differed between the 2 studies (54% vs 77%), as did the distribution of several baseline parameters between responders and nonresponders (sex, diabetes, previous stroke). The validation cohort showed a higher overall median baseline LVEF, shorter median QRS duration, fewer patients with a known HF etiology, and better recurrence-free survival in responders.
This study as several limitations, namely, its retrospective design, the small percentage of women (19%) and nonresponders (22.6%), the unavailability of cardiac magnetic resonance imaging, the lack of AF burden quantification during follow-up, nonstandardized LVEF estimation (66% biplane, 33% visual, 1% other), and the imputation of LAVI in a small number of patients.
In conclusion, in this external cohort, the Antwerp score showed modest performance in identifying patients with LVEF recovery after catheter ablation. QRS duration and the lack of a known etiology of HF were confirmed as excellent predictors of AiCM. The study meets ethical standards and was approved by the ethics committee of our institution. All patients signed the informed consent form.
FUNDINGNo funding was received for the current study.
AUTHORS’ CONTRIBUTIONST. Serban: data gathering and cleaning, statistical analysis, manuscript drafting and revision. J. du Fay du Lavallaz: manuscript proofing, statistical analysis proofing. D.C. Barker: data gathering. C. Sticherling: manuscript review. M. Kühne and P. Badertscher: project supervision, manuscript review; both contributed equally and should be considered joint last authors.
CONFLICTS OF INTERESTNo conflicts of interest are reported for the current study. T. Serban: research funding from the Swiss Academy of Medical Sciences and the ‘Gottfried & Julia Bangerter-Rhyner’ Foundation. P. Badertscher: research funding from the University of Basel, the “Stiftung für Herzschrittmacher und Elektrophysiologie”, the “Freiwillige Akademische Gesellschaft Basel” and Johnson&Johnson, all outside the submitted work and reports personal fees from Abbott. C. Sticherling: member of Medtronic Advisory Board Europe and Boston Scientitic Advisory Board Europe, received educational grants from Biosense Webster and Biotronik and a research grant from the European Union's FP7 program and Biosense Webster and lecture and consulting fees from Abbott, Medtronic, Biosense Webster, Boston Scientific, Microport, and Biotronik all outside the submitted work. M. Kühne: personal fees from Bayer, Böhringer Ingelheim, Pfizer BMS, Daiichi Sankyo, Medtronic, Biotronik, Boston Scientific, Johnson&Johnson, and Roche; grants from Bayer, Pfizer, Boston Scientific, BMS, Biotronik, Daiichi Sankyo, all outside the submitted work. J. du Fay du Lavallaz: research funding from the University of Basel and from the Swiss Heart Foundation. Other authors have nothing to declare.