The article "Differentiating Between Atrial Flutter and Atrial Fibrillation Using Right Atrial Bipolar Endocardial Signals" published by Isa et al1 might pose a surprising question for many readers. Is it really that difficult to distinguish between atrial flutter and atrial fibrillation, or is it that electrophysiologists like to complicate the issue?
Strictly speaking, the approach taken in the study could be criticized on the basis of the bias inherent in the selection of the patients, since if patients were selected according to presentation of atrial fibrillation with an "organized electrical pattern" it is not surprising that the variation in the intervals between the electrograms was not indicative of atrial fibrillation in that group. The study also lacks quantification of the incidence of such an organized pattern of atrial fibrillation in the right atrium to allow assessment of the general applicability of the values of sensitivity and specificity calculated based on the study group. However, aside from methodologic questions, which can always be raised, the study addresses an underlying problem that is of major theoretical and practical interest and that can be summarized in the question expressed in the title of this editorial: what exactly is atrial fibrillation and how do we diagnose it?
Surprising as it may seem, there is no simple and accurate electrophysiologic definition of atrial fibrillation. For instance, the definition offered in the August 2006 edition of the American Heart Association/American College of Cardiology/European Society of Cardiology guidelines2 is limited to a description of the pattern of irregular atrial waveforms in the electrocardiogram (ECG), with a reference to the book published by Bellet3 in 1971. Atrial fibrillation is also defined in this way in many studies from the electrophysiology literature. It is true that at least the latest guidelines draw attention to the fact that an irregular ventricular rate is not diagnostic of atrial fibrillation and that atrial flutter can generate this irregularity as a result of irregular atrioventricular conduction patterns. But, in continuing to focus on the ECG pattern as the only diagnostic criterion of atrial fibrillation, have we not made any progress at all in 35 years? And to what extent do we understand the underlying causes of this irregular ECG pattern?
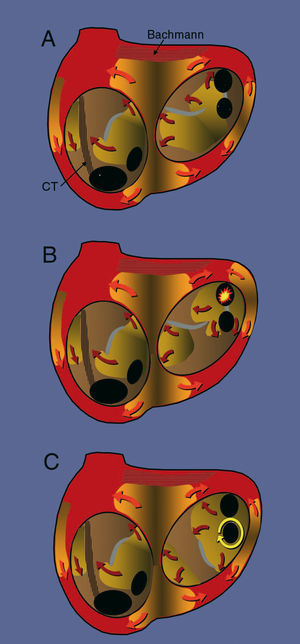
Atrial fibrillation has always been considered a reflection of the fragmentation of atrial activation into multiple wavelets of varying width and spread. In the 1950s, it was suggested that there was a focal origin for this activation during atrial fibrillation, implying that the fragmentation of activation would be due to the inability of the atrial myocardium to conduct regularly at very high frequencies4 (Figure 1). Impulse conduction would encounter refractory and anisotropic regions that would produce functional blocks with a variable spatial distribution, leading to fragmentation of activation into multiple fronts or waves. This is known as fibrillatory conduction. This hypothesis of the focal origin of atrial fibrillation was soon sidelined by the hypothesis of sustained reentry of multiple wavelets proposed by Moe et al5 and confirmed in 1985 by Allessie et al6 using advanced atrial endocardial mapping. According to this hypothesis, atrial fibrillation is a chaotic reentry (complexly determined) with multiple simultaneously active wavefronts (Figure 1) that can be sustained indefinitely as long as the preparation or the fibrillating organ is sufficiently large, the refractory period is short and variable (dispersion of refractoriness), and the conduction velocity is slow. Allessie et al found that the coexistence of at least 6 activation fronts was necessary in order for atrial fibrillation to be sustained indefinitely. During mapping of atrial fibrillation, they occasionally observed new activation fronts that indicated the presence of foci,6,7 but these apparent anomalies were explained as the result of 3-dimensional reentry.8
Figure 1. Schematic representation of the 3 mechanisms described for atrial fibrillation. The endocardial surface is shown through the mitral and tricuspid valves, revealing the openings of the inferior vena cava (IVC), the coronary sinus (CS), and the left pulmonary veins (PV). The surfaces colored in orange represent the activation fronts and the arrows indicate the direction in which the wavefront is propagated, with a lighter leading edge and a darker refractory wake. The grey areas represent lines of functional block. The hatched regions represent anisotropic fiber bundles: the crista terminalis (CT) in the right atrium and Bachmann's bundle in the left. A) Activation through multiple wavelets, with no localized point of origin, maintained by the dynamics of the activation fronts themselves. B) Focal mechanism in which irregular (fibrillatory) activation has its origin in a high frequency automatic focus (yellow star) in a pulmonary vein. C) Rotor anchored in the region of the left inferior pulmonary vein that drives activation and also produces fibrillatory conduction.
Studies of atrial activation during atrial fibrillation in humans have revealed a notable complexity, making it difficult to arrive at a simple definition using endocardial recordings. Already in 1978, Wells et al9 published a study in patients with atrial fibrillation following heart surgery in which isolated atrial electrograms were recorded over periods of up to 15 minutes via electrodes sutured in an unspecified region of the "atrial epicardium." The intervals between the atrial potentials ranged from highly organized, with well-defined isoelectric intervals between the complexes (1/3 of all cases), to completely disorganized recordings in which it was impossible to define individual complexes (continuous fragmented activity). However, in all cases, analysis of a large number of complexes over time revealed variation in the intervals between individual complexes of up to 100 ms with changes from highly organized electrograms to a disorganized pattern and vice versa. This contrasted with the exquisite organization and regularity of the recordings obtained by the same group during episodes of atrial flutter in the same clinical context.10 Similar observations were made by Konings et al11 using high-density activation mapping of the anterior right atrium performed during atrial fibrillation induced during heart surgery. Those authors observed relatively uniform activation at this site, with 1 or 2 activation fronts in 72% of the patients, although there was a variation in the interval between cycles of greater than 40 ms. The presence of multiple activation fronts (waves) could only be demonstrated in 28% of cases, while the majority of the patterns indicated passive activation that was more or less irregular and originated in some point outside the atrium. One limitation of that study was the short analysis time of 12 seconds (actually very similar to that used in the study of Iza et al1). A longer period of recording would probably increase the possibility of detecting greater variation in the local intervals.9 The organization of activation in the anterior right ventricle is favored by the conduction barrier represented by the crista terminalis12-14 and the openings of the vena cava and tricuspid valve, meaning that this region is probably quite unrepresentative of the general mechanism of atrial fibrillation. The organization of electrical activity in the right atrium can be extreme in cases of atrial fibrillation with defective interatrial conduction.15
The recent resurgence of the focal hypothesis of atrial fibrillation is now well known through the work of Haïssaguerre et al,16 which has been confirmed by other authors,17 demonstrating induction of atrial fibrillation following rapid discharges from the pulmonary veins and, less frequently, from other venous and atrial structures.18 But this observation is complemented by others related not to the onset but rather to the maintenance of atrial fibrillation. In 1992, Schuessler et al19 demonstrated in dogs that localized reentry could provoke irregular (fibrillatory) activation of the atria that was indistinguishable from atrial fibrillation unless very detailed mapping of the origin of the activation was performed. Later, the group led by Jalife demonstrated using optical mapping in sheep that a stable rotor could sustain atrial fibrillation20 (Figure 1). These rotors were mainly located in the left atrium, while the right atrium tended to be passively and irregularly activated through the appearance of lines or regions in which high frequency activation was blocked. Local ablation of the anchor point of the rotor can prevent induction of atrial fibrillation in some experimental models21; however, other models of functional reentry in the atrium have confirmed that rotors can migrate from one zone to another in both atria,22 making the proposed "focal" ablation of these anchor points highly debatable.
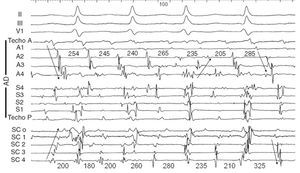
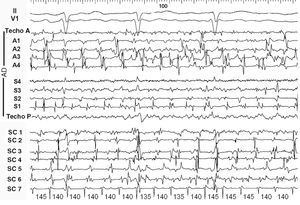
Returning to the diagnosis of atrial fibrillation, we find that endocardial recordings in patients with irregular atrial activation and a changeable configuration in the ECG can exhibit highly variable patterns. It is easy to record electrograms from multiple points in the right atrium with multielectrode catheters, but the activity of the left atrium is usually only recorded from the coronary sinus. In some cases relatively organized activity is recorded in both the right atrium and the coronary sinus, but with continuously changing sequences (Figure 2); however, in other cases with highly fragmented activity throughout the right atrium and in the recordings from the coronary sinus, a highly regular and very rapid activity can be recorded in a highly localized point, perhaps indicating the presence of a microreentrant circuit (rotor) with fibrillatory conduction to both atria (Figure 3).
Figure 2. Electrocardiogram (ECG) and endocardial recordings in atrial fibrillation 1. Moving from top to bottom, the figure shows leads II, III, and V1, which record low voltage disorganized atrial waves, followed by bipolar recordings from the right atrium (RA) that cover the anterior wall from the roof (Ant Roof) to the most inferior portion (A4) centimeter by centimeter, and the septal wall from the most inferior portion (S4) to the most superior (Post Roof), centimeter by centimeter. The lower part of the figure shows the recordings in the coronary sinus from the ostium (CSo) to the distal coronary sinus (CS4), centimeter by centimeter. The numbers indicate the intervals between the complexes. The arrows indicate changes in the direction of activation. It can be seen that although there are wide isoelectric intervals between the complexes, the intervals and sequences change continually.
Figure 3. Electrocardiogram (ECG) and endocardial recordings in atrial fibrillation 2. In the upper part of the figure, leads II and V1 show disorganized atrial activity. All of the recordings from the right atrium (RA) and coronary sinus (CS) display complete disorganization, with multiple deflections and a nonexistent baseline at numerous points. The only exception is in the most distal CS (CS7) recordings, which display perfectly regular complexes at very high frequency (140-ms cycle, equivalent to 430/min), consistent with regular, highly localized reentrant activity (rotor) in the left atrium as a driver of activation.
Antiarrhythmia drugs would tend to increase the organization of the atrial fibrillation, either by prolonging the refractory period or by widening the radius of the pivot point for the reentrant wavefront,23 with a reduction in the number of small rotors. The drugs can also increase the block at the crista terminals,24 an effect that would accentuate even further the organization of activation. In fact, the appearance of flutter in patients with atrial fibrillation treated with antiarrhythmic drugs is no more than a consequence of this organization of reentry.
What can we use then to establish a differential diagnosis between atrial fibrillation and flutter, to allow us to obtain an automatic diagnosis? When using individual electrograms, the answer lies in the recording time. If sufficiently long recordings are used, lasting at least 30 seconds, it is very rare not to observe a variation of ≥ 50 ms between the atrial fibrillation potentials, while in atrial flutter the reverse is true. The use of a long detection period is perfectly possible in this situation, since these arrhythmias are rarely life threatening; in addition, it would avoid unnecessary and potentially arrhythmogenic interventions in episodes of self-limiting tachycardia. Sufficient time for recording and examination is also central to differential diagnosis of atrial flutter and atrial fibrillation, where transitions between one arrhythmia and the other are also possible and the use of various recording points over an extended period will sooner or later reveal the irregularity and changes in sequence associated with atrial fibrillation. Similarly, detailed analysis of the pattern of atrial waveforms in the ECG allows recognition of their irregularity and changing morphology during atrial fibrillation in cases where their relative organization suggests a possible diagnosis of atrial flutter.
Finally, how do we refer to a rhythm that generates organized activity in one atrium and disorganized activity in the other? Should we develop terms like "localized flutter with fibrillatory conduction" or "atrial fibrillation with a localized source?" There are no answers to these questions, and the analysis of each case will have to carry with it a descriptive definition of the mechanism observed. If fibrillatory conduction is an indicator of diffuse defects in atrial conduction, the distinction between this and multiple reentry would be of little practical use, because both would indicate profound electrophysiologic dysfunction. For the time being, only the perfect organization of activation in both atria that characterizes atrial flutter25 opens the way for treatment by cardioversion using programmed pacing and/or curative treatment by catheter ablation of an isthmus critically located in the circuit.
Correspondence: Dr. F. García-Cosío.
Servicio de Cardiología. Hospital Universitario de Getafe.
Ctra. de Toledo, km. 12,5. 28905 Getafe. Madrid. España.