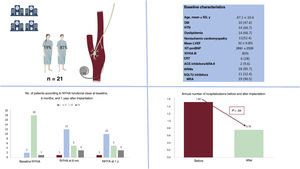
Baroreflex activation therapy (BAT) reduces sympathetic nervous system activity and boosts the parasympathetic nervous system. BAT has been shown to improve quality of life, functional class, and natriuretic peptide levels in patients with heart failure (HF) and reduced ejection fraction (HFrEF) who are not eligible for cardiac resynchronization therapy (CRT) and remain symptomatic despite pharmacologic treatment.1,2 Little, however, has been published on the effectiveness or safety of BAT in real-world settings.3,4 In addition, the latest European and American HF guidelines do not establish a level of recommendation for BAT.5,6 The aims of this study were to describe the clinical profile of the first cohort of patients to be implanted with Barostim Neo System (CVRx, USA) in Spain and to evaluate the safety and effectiveness of this device in clinical practice.
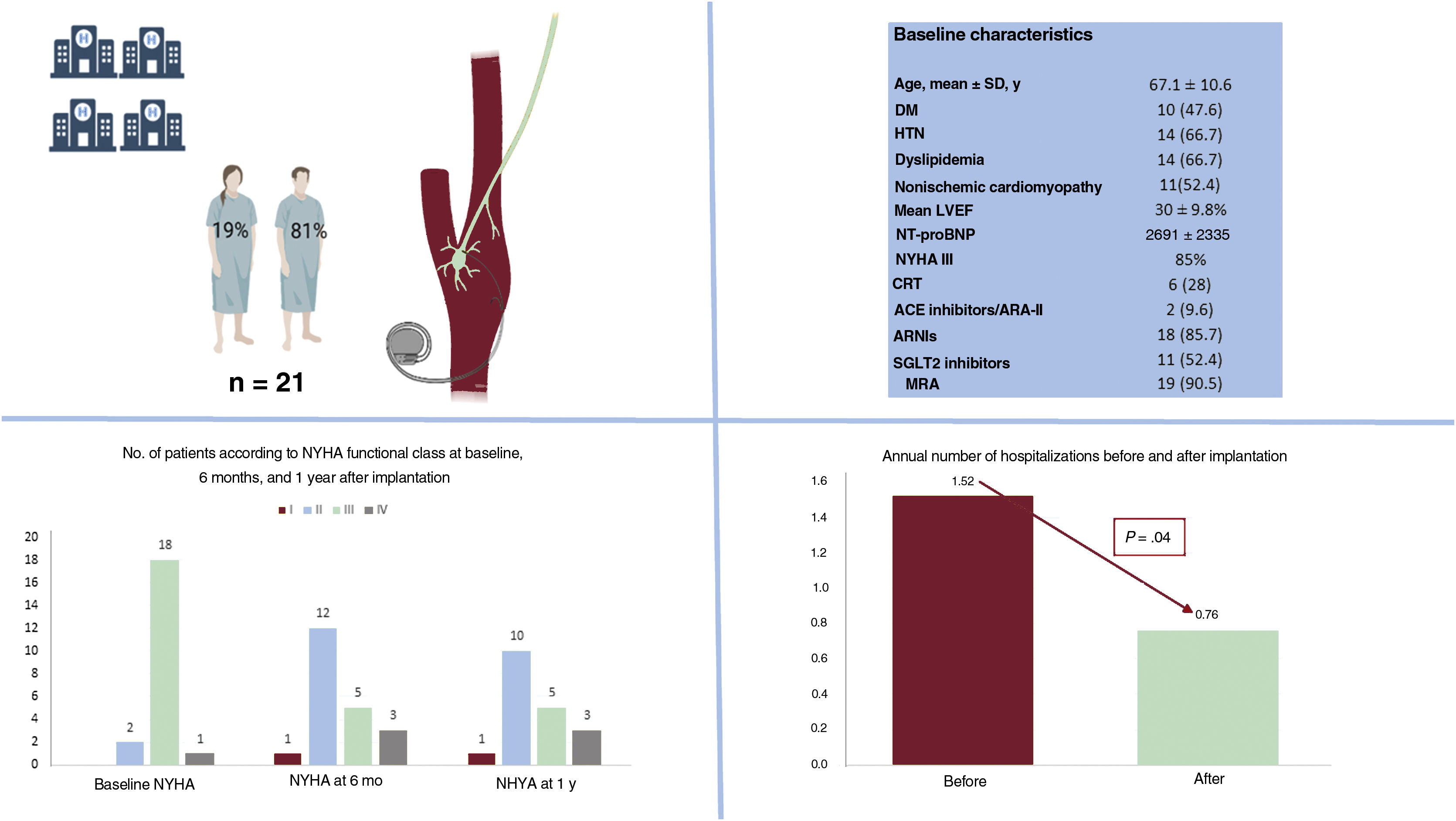
Informed consent was deemed unnecessary by the ethics committee of the coordinating center due to the observational nature of the study. We conducted a retrospective cohort study of 21 consecutive patients with HFrEF who received an implantable Barostim Neo System at a Spanish hospital between February 2017 and December 2021 and had follow-up data for 1 year. The hospitals and principal investigators are listed in the appendix. The patients’ baseline characteristics are summarized in table 1 and compared with those of the patients in the intervention group in the pivotal BeAT-HF clinical trial.2 The device was activated 17.9±8.2 days after surgery. The mean amplitude achieved was 6.6±1.3mA, with a mean pulse width of 129±12μs and a mean therapy frequency of 40 pulses/s per patient. Heart rate and blood pressure decreased, but not significantly, after activation of the BAT device. The mean number of annual hospitalizations for HF after implantation of the Barostim Neo System was 1.52, which was significantly lower than the previous year (0.76, P=.042). A trend towards improved New York Heart Association (NYHA) functional class was also observed after 1 year of follow-up (P=.054). There were no differences in the annual number of outpatient intravenous diuresis visits before and after implantation (24 vs 20, P=.450). There were also no differences between baseline and follow-up at 1 year for mean left ventricular ejection fraction (LVEF) (30%±9.8% vs 31.8%±11.2%, P= .689), mean left ventricular end-diastolic diameter (58.79±6.2 vs 60.6±5mm, P=.325), or median N-terminal pro–B-type natriuretic peptide (NT-proBNP) (1119 [interquartile range, 450-2763] vs 1149 [499-4798] pg/mL; P=.756) (figure 1). None of the patients developed local complications, and they had a similar number of appropriate implantable cardioverter-defibrillator therapies in the year before and after implantation (0.53 vs 0.58; P=.902). Three patients (14.2%) developed minor BAT-related complications (hoarseness, dry cough, and neck pain) during the 12 months of follow-up. None of the complications required treatment discontinuation and all were resolved by adjusting the stimulation parameters. Three patients (14.2%) died during follow-up: 2 of refractory HF and 1 of stroke unrelated to BAT.
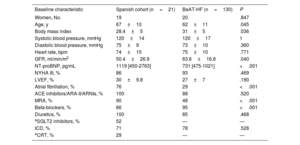
Comparison of baseline characteristics between Spanish cohort and patients in BeAT-HF clinical trial
| Baseline characteristic | Spanish cohort (n=21) | BeAT-HF (n=130) | P |
|---|---|---|---|
| Women, No. | 19 | 20 | .847 |
| Age, y | 67±10 | 62±11 | .045 |
| Body mass index | 28.4±5 | 31±5 | .036 |
| Systolic blood pressure, mmHg | 120±14 | 120±17 | 1 |
| Diastolic blood pressure, mmHg | 75±9 | 73±10 | .360 |
| Heart rate, bpm | 74±15 | 75±10 | .771 |
| GFR, ml/min/m2 | 50.4±26.9 | 63.6±16.8 | .040 |
| NT-proBNP, pg/mL | 1119 [450-2763] | 731 [475-1021] | <.001 |
| NYHA III, % | 86 | 93 | .469 |
| LVEF, % | 30±9.8 | 27±7 | .190 |
| Atrial fibrillation, % | 76 | 29 | <.001 |
| ACE inhibitors/ARA-II/ARNIs, % | 100 | 88 | .520 |
| MRA, % | 90 | 48 | <.001 |
| Beta-blockers, % | 66 | 95 | <.001 |
| Diuretics, % | 100 | 85 | .468 |
| aSGLT2 inhibitors, % | 52 | — | — |
| ICD, % | 71 | 78 | .528 |
| aCRT, % | 29 | — | — |
ACE, angiotensin-converting enzyme; ARA-II, angiotensin II receptor antagonists; ARNIs, angiotensin receptor neprilysin inhibitors; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association functional class; SGLT2, sodium-glucose cotransporter type 2.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Comparison of annual events before and after implantation of the Barostim Neo System: mean number of hospitalizations for heart failure and mean NYHA functional class. Main baseline characteristics. ACE, angiotensin-converting enzyme; ARA-II, angiotensin II receptor antagonists; ARNIs, angiotensin receptor neprilysin inhibitors; CRT, cardiac resynchronization therapy; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; HTN, hypertension; MRA, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; SD, standard deviation; SGLT2, sodium-glucose cotransporter type 2.
This is the first study to present real-world data on BAT for HFrEF in Spain. Use of the Barostim Neo System in a population with symptomatic HF despite optimal medical management was associated with a reduction in hospitalizations for HF and a trend towards improved NYHA functional class at 1 year, with no significant complications. Our findings corroborate previous reports showing that BAT is a safe option for patients with HFrEF1,2 and highlight the prognostic benefits of this treatment (in our case, fewer admissions for HF). No improvements were observed for natriuretic peptide levels, NYHA functional class, or LVEF at 1 year, but this is probably due to the small sample size and short follow-up period. In this first cohort of patients to receive BAT in Spain, the reduction in hospitalizations for HF is significant, as, compared with patients in the BeAT-HF trial,2 they were considerably older and had worse kidney function, a higher prevalence of atrial fibrillation, and higher NT-proBNP levels, generally indicating more advanced HF despite optimal pharmacologic and device therapy. (Although BAT is indicated for patients who are not considered eligible for CRT, almost one-third of the patients in our cohort were receiving CRT.) We also consider that BAT could be an attractive alternative for patients intolerant to beta-blockers (34% of the present cohort). In such cases, the device settings were adjusted to achieve a stimulation energy similar to that observed in patients who tolerate these drugs (6.8 vs 6.9 mA, P=0.449). This potential benefit, however, needs to be confirmed in studies designed to specifically test this hypothesis. BAT might also be useful in patients with chronic renal failure and diuretic resistance, as we observed a trend towards a reduced need for loop diuretics and lower doses of these drugs at 1 year. There was also some stabilization of glomerular filtration at this point. From a mechanistic perspective, the immediate inhibition of the sympathetic nervous system achieved by BAT could lead to greater blockade of the renin-angiotensin-aldosterone system, ultimately, resulting in cardiorenal protective effects. Again, this theory needs to be tested.
In conclusion, the 1-year results of this real-world Spanish study of BAT in patients with HFrEF who remain symptomatic despite optimal medical management are positive, as the patients required significantly fewer hospitalizations for HF, showed a trend towards improved NHYA functional class, and experienced no major complications. Long-term data from ongoing clinical trials and new findings from clinical practice will help position this promising technique in the treatment algorithm for patients with HFrEF.
FUNDINGThe authors declare that they did not receive any funding for this study.
ETHICAL CONSIDERATIONSThis study was approved by the ethics committee of the coordinating center. Informed consent was not deemed necessary due to the observational, retrospective nature of the study. As a limitation, the SAGER (Sex and Gender Equity in Research) guidelines were not applied.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used in this study.
AUTHORS’ CONTRIBUTIONSD. Cordero Pereda and C. de Rueda Panadero contributed equally to this work as first authors and prepared the initial draft of the manuscript. D. Cordero Pereda and J. Álvarez-García conceived and designed the study and analyzed the data. C. de Rueda Panadero, J. de Juan Bagudá, M. Gómez Bueno, and A. Robles-Mezcua collected and interpreted the data and participated in the critical review and discussion. All authors approved the final version of the manuscript for publication.
CONFLICTS OF INTERESTThe authors do not have conflicts of interest in relation to this article.
The authors guarantee that the following researchers are responsible for the data contained in this work: David Cordero Pereda, Clemencia de Rueda Panadero, Susana del Prado Díez, Marta Jiménez-Blanco Bravo, Paloma Remior Pérez, Sandra González Martín, Claudio Gandarias Zúñiga, José Luis Zamorano Gómez, and Jesús Álvarez-García from Hospital Universitario Ramón y Cajal in Madrid; Javier de Juan Bagudá and Rafael Salguero Bodes from Hospital Universitario 12 de Octubre in Madrid; Manuel Gómez Bueno and Javier Segovia Cubero from Hospital Universitario Puerta de Hierro in Madrid; Ainhoa Robles-Mezcua and José Manuel García Pinilla from Hospital Universitario Virgen de la Victoria in Malaga; and José Cordero Guevara from Instituto de Investigación Sanitaria Bioaraba in Vitoria.