Keywords
INTRODUCTION
Primary cardiac tumors are rare, with a post mortem incidence of 0.001%-0.028%.1 The symptoms associated with them are very non-specific; electrocardiogram findings and the results of physical examinations and chest x-rays are usually inconclusive. Imaging techniques therefore play an important role in the detection and differential diagnosis of cardiac masses.
CASE REPORT
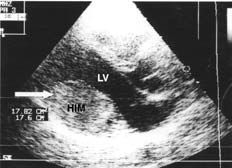
The patient, who practiced sport habitually, was a 33 year-old man who came for consultation regarding palpitations and exercise-induced dyspnea--symptoms he had noticed for 3 months. A physical examination and chest x-ray were both normal. An electrocardiogram (ECG) showed deep, negative T waves in II, III, aVF, V5, and V6; these had been noticed in an ECG some 8 years earlier. At that time the patient was studied for precordial pain; an arrhythmic cause was ruled out by Holter-ECG. However, echocardiography was not performed. Transthoracic echocardiography (TTE) (Figure 1) now showed a homogeneous intracardial mass of 45*55 mm on the posterolateral wall of the left ventricle. The echodensity of this mass was similar to that of the myocardium. Although the mass protruded into the cavity, left ventricular systolic function was not compromised. The patient's biochemical, hemogram, and coagulation results were normal. Tumor markers were negative. Holter-ECG detected isolated ventricular and atrial extrasystoles; no tachyarrhythmia was recorded.
Figure 1. Transthoracic echocardiogram (parasternal plane, long axis) showing a homogeneous intramyocardial mass (HIM, marked with an arrow) on the posterior wall of the left ventricle (LV), protruding into the cavity.
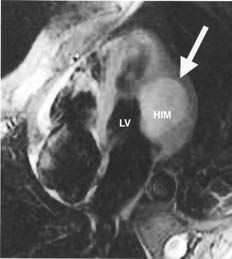
Magnetic resonance (Figure 2) confirmed the presence of an intramural mass with no evidence of myocardial or pericardial infiltration. The mass was hyperintense during the STIR sequence, mildly hyperintense in T1, and isointense or minimally hypointense in T2. Early, heterogeneous highlighting was seen after the injection of gadolinium.
Figure 2. Magnetic resonance during the STIR sequence showing a hyperintensive image of the homogeneous intramyocardial mass (HIM, marked with arrow) on the lateral wall of the left ventricle (LV).
Ventriculography showed a filling defect in the lower part of the ventricle. Coronary angiography showed irrigation of the mass via the obtuse marginal branch and the right coronary artery. Thoracoabdominal computed tomography detected neither adenopathy nor other masses.
A benign heart tumor interfering with left ventricular filling was suspected and surgery scheduled. Following left ventriculotomy, the mass was partially resected and the posteromedial papillary muscle reimplanted.
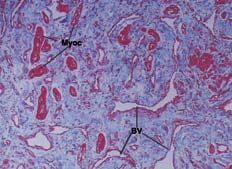
Anatomopathological examination (Figure 3) showed an irregular distribution of histological elements, including disordered, hypertrophied muscle fibers containing myocytes with large nuclei, surrounded by fibrous and adipose tissue. The final diagnosis was a hamartoma composed of mature cardiomyocytes.
Figure 3. Anatomopathological section showing hypertrophied myocytes (Myoc). These cells are disordered. Abundant fibrous connective tissue and malformed blood vessels (BV) can also be seen.
Following surgery, Holter-ECG detected a very weak ventricular extrasystole. Ergometry was negative (patient score 11 METS). The patient followed a cardiac rehabilitation program and has been asymptomatic and has suffered no post-operative problems for three years.
DISCUSSION
The low cost and innocuous nature of echocardiography make it the technique of choice in initial studies of heart masses. The technique can localize such masses, define their shape, size, mobility, and point of anchorage, and determine whether they are solid or cystic in nature.
Magnetic resonance supplies structural and hemodynamic information since it offers both static and dynamic sequences, and images can be acquired from an unlimited number of planes and projections. Tissue characterization via images potentiated in T1 and T2 complete this information. Slightly increased intensity in T1 is seen in tissues with fibrous or muscular contents, but never where there is adipose tissue.2 Hypointensity in T2 rules out that the mass contains liquid. These tissue characteristics, plus the absence of infiltration, first suggested the present mass might be a fibroma. However, the images obtained after gadolinium injection showed good vascularization. This suggested it not to be a fibroma since these tumors have low metabolic requirements2; at this point it was believed the mass might be a hemangioma or a malignant tumor. Coronary angiography was performed to define the vascular supply and to help indicate the surgical technique to follow. Coronary angiography also supplies data on the presence of obstructive arterial disease, and on the vascular malformations seen in rare cases of intramyocardial dissecting hematoma.3,4 It can also be used to ratify a diagnosis of hemangioma.5
A hamartoma is a benign overgrowth of the mature, differentiated cells of the organ in which it is found. However, these cells are disorganized. The mass results from the anomalous development of embryonic cells.
Focal hypertrophic cardiomyopathy (FHC) and rhabdomyoma also involve hypertrophied myocytes, but with different histological and pathogenic characteristics. A family background and a preference for the septal region tend to be associated with FHC, whereas presentation during infancy and an association with Bourneville's disease are related to rhabdomyoma. The definitive diagnosis is anatomopathological.
The first descriptions of hamartomas composed of mature heart cells appeared in 1998.6,7 The intracardiac location of such tumors has been reported in small groups of patients.6-13 An essential characteristic of cardiac hamartomas is the presence of hypertrophied myocytes lying in a disordered fashion and mixed with vascular, fibrous and fatty tissue in different proportions. This varied histological presentation has led to a certain nomenclatural confusion. Hamartomas can appear on their own, but on rare occasions can be multiple. They preferentially develop on the ventricle wall but have been reported on valve tissue.9
One of the most common manifestations of these tumors is ventricular tachycardia in children and young people10-13; a case of a 2 year-old was reported in this journal.13 Treatment is surgical and good short-term results are usually achieved. The present patient noticed palpitations; ventricular extrasystoleS were detected but no tachycardia was recorded.
The presentation of hamartomas in young adults, and their slow growth, suggests a possible congenital origin with hypertrophy and development of the mass in the first years of life. In the present case, the recording of electrical anomalies 8 years before the current events suggests this possibility. Other authors have described congenital fibrous cardiac tumors (not associated with complex hereditary problems) in infancy, adolescence and early adulthood.2