The aim of this study was to analyze the clinical profile, management, and prognosis of ST segment elevation myocardial infarction-related cardiogenic shock (STEMI-CS) requiring interhospital transfer, as well as the prognostic impact of structural variables of the treating centers in this setting.
MethodsThis study included patients with STEMI-CS treated at revascularization-capable centers from 2016 to 2020. The patients were divided into the following groups: group A: patients attended throughout their admission at hospitals with interventional cardiology without cardiac surgery; group B: patients treated at hospitals with interventional cardiology and cardiac surgery; and group C: patients transferred to centers with interventional cardiology and cardiac surgery. We analyzed the association between the volume of STEMI-CS cases treated, the availability of cardiac intensive care units (CICU), and heart transplant with hospital mortality.
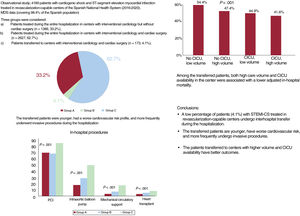
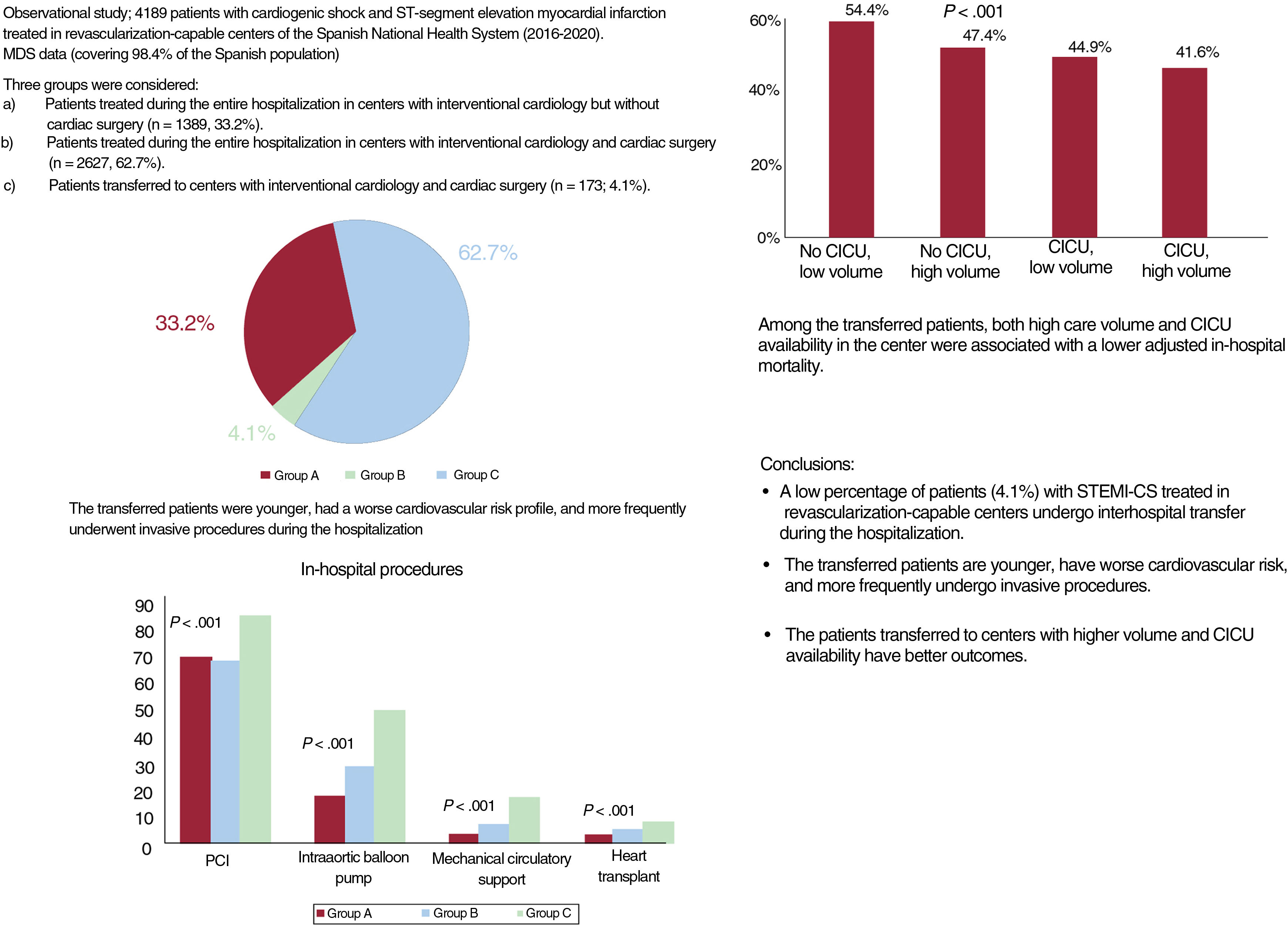
ResultsA total of 4189 episodes were included: 1389 (33.2%) from group A, 2627 from group B (62.7%), and 173 from group C (4.1%). Transferred patients were younger, had a higher cardiovascular risk, and more commonly underwent revascularization, mechanical circulatory support, and heart transplant during hospitalization (P<.001). The crude mortality rate was lower in transferred patients (46.2% vs 60.3% in group A and 54.4% in group B, (P<.001)). Lower mortality was associated with a higher volume of care and CICU availability (OR, 0.75, P=.009; and 0.80, P=.047).
ConclusionsThe proportion of transfers in patients with STEMI-CS in our setting is low. Transferred patients were younger and underwent more invasive procedures. Mortality was lower among patients transferred to centers with a higher volume of STEMI-CS cases and CICU.
Keywords
Cardiogenic shock (CS) continues to cause considerable mortality.1 In this setting, early revascularization in acute coronary syndrome (ACS) is the only therapeutic approach shown to significantly affect outcomes.2 Some structural variables of the treating centers, such as the annual number of CS cases,3 cardiac intensive care unit (CICU) availability,4 and shock team presence,5,6 are significantly associated with improved clinical outcomes. Accordingly, the current guidelines recommend the centralized management of these patients in high-level and high-volume centers with full-time availability of interventional cardiology laboratories and circulatory assist devices.7 In addition, CS treatment should be organized in care networks comprising centers of various levels. This approach represents a considerable organizational challenge that requires a reinforcement of staffing numbers in referral centers8 and highly complex interhospital transfer systems. In Spain, the regional management of CS is widely heterogeneous, and there are no reliable data on the number of patients with CS transferred between centers in routine practice or their clinical profile, management, or outcomes vs nontransferred patients with CS. Thus, the objective of the present study was to analyze the proportion, clinical characteristics, and clinical course of patients with CS transferred between centers among a large series of patients with CS due to ST-segment elevation acute myocardial infarction (STEMI) treated in revascularization-capable centers (RCCs) in the Spanish National Health System (SNHS).
METHODSStudy design and populationThis retrospective observational study included patients with a primary or secondary diagnosis of CS (ICD-10 code: R57.0) and STEMI (ICD-10 codes: 21.01, I21.02, I21.09, I21.11, I21.19, I21.21, I21.29, or I21.3), both present at admission. We enrolled patients discharged between 2016 and 2020 from SNHS centers with on-site cardiac catheterization (type 3 or 4 centers according to the RECALCAR classification) (table 1). Data were obtained from the minimum data set (MDS),9 an administrative database that includes demographic and clinical information on all patients discharged from all publicly funded hospitals belonging to the SNHS, which covers 98.4% of the Spanish population.
Hospital type by RECALCAR classification
| Group | Characteristics |
|---|---|
| 1 | Units without hospital beds assigned to cardiology |
| 2 | Units with hospital beds specifically assigned to cardiology, without a cardiac catheterization laboratory |
| 3 | Units with hospital beds assigned to cardiology, with a cardiac catheterization laboratory, without an in-hospital cardiovascular surgery unit |
| 4 | Units with hospital beds assigned to cardiology, with an in-hospital cardiac catheterization laboratory and cardiovascular surgery unit |
| 5 | Units without beds assigned to cardiology with cardiac catheterization activity and/or cardiovascular surgery |
Multiple admissions related to an interhospital transfer were considered a single health care episode. The clinical outcomes of transferred patients were assigned to the referral hospital receiving the transfer. We excluded episodes occurring in patients younger than 35 years, voluntary discharges or those with unknown destinations, episodes with hospital stay durations less than 1 day, and discharges to home. The ICD-10 codes used to identify comorbidities and complications are shown in tables 1, 2, and 3 of the supplementary data. The codes used to identify the procedures performed during the episode are shown in table 4 of the supplementary data.
CS episodes due to STEMI (STEMI-CS) were divided into 3 groups: group A, patients admitted to a hospital with an interventional cardiology unit but without cardiac surgery (type 3 centers) and discharged from the same hospital, without an interhospital transfer; group B, patients admitted to hospitals with an interventional cardiology unit and cardiac surgery (type 4 centers) and discharged from the same hospital, without an interhospital transfer; and group C, patients who, although admitted to a hospital with an interventional cardiology unit, with or without cardiac surgery, were transferred to another hospital with on-site interventional cardiology and cardiac surgery.
Hospital characteristicsAll hospitals included in this study had an in-hospital critical care unit, either a CICU or a general intensive care unit (ICU). For the purpose of this study, a CICU was considered to be a unit that treated critically ill cardiovascular patients, including those who required invasive mechanical ventilation, and that was administratively attached to a cardiology department.10 CICU availability data were obtained from a survey conducted by the Ischemic Heart Disease and Acute Cardiovascular Care Association of the Spanish Society of Cardiology.10 Data on the availability of heart transplant (HTx) programs were obtained from the National Transplant Organization of Spain.
Ethical responsibilities and statistical analysisDue to the characteristics of the study (based on an anonymized administrative database, with a large-scale approach and retrospective design), informed consent was not required from patients.
Continuous variables are expressed as mean ± standard deviation or as median [interquartile range]. Categorical variables are expressed as percentages. Categorical variables were analyzed using the chi-square test or Fisher exact test and differences in continuous variables were compared using a 2-sided t test or Mann-Whitney U test.
Multilevel logistic regression models were specified and adjusted for the main endpoint analyzed, in-hospital mortality. These models were based on the Centers for Medicare and Medicaid Services (CMS) method for acute myocardial infarction,11 adapted to the data structure of the MDS after grouping of the secondary diagnoses according to the quality and research condition categories of the Agency for Healthcare Research and Quality, which are updated every year.12 We included variables with a significant association in univariable analysis with an odds ratio (OR) >1.00. The stepwise backward elimination technique was used to estimate the adjusted models, with significance thresholds of P<.05 for inclusion and of P≥.10 for elimination. Model discrimination was evaluated using the receiver operating characteristics (ROC) curve and the corresponding area under the curve (AUROC). The calibration was analyzed graphically after we grouped the patients in deciles according to predicted probabilities and tabulated the predicted probabilities against the observed probabilities. Risk-standardized mortality rates were calculated from specific models.13,14
The association between in-hospital mortality and hospital characteristics was analyzed by considering the following independent variables in multilevel logistic regression models: the annual STEMI case number in each hospital, CICU availability, and HTx programs. The threshold of the annual STEMI-CS case volume for distinguishing low- and high-volume centers was calculated using a k-means clustering algorithm, which obtains maximum and minimum densities within a cluster. To minimize the selection bias in the outcome comparison, we assessed the impact of the structural characteristics of the hospitals on in-hospital mortality using propensity score matching (k-nearest neighbors matching option, psmatch2 in STATA) and by considering the same variables used for the risk adjustment models. Matching was performed in a 1:1 ratio and without replacement. All comparisons were 2-sided and differences were considered significant at P<.05. Odds ratio and their corresponding 95% confidence intervals (95%CIs) were also calculated. All analyses were performed with STATA 16.0 (Stata Corp, United States) and SPSS 20.
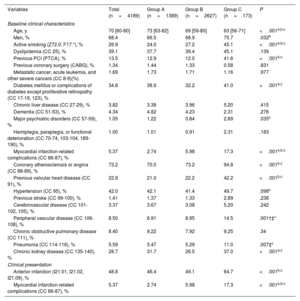
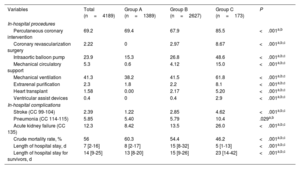
RESULTSIn total, 4437 STEMI-CS episodes were identified in patients admitted to RCCs. After application of exclusion criteria, the study population comprised 4189 episodes (94.4%) (figure 1 of the supplementary data): 1389 episodes (33.2%) occurred in group A, 2627 (62.7%) in group B, and 173 (4.1%) in group C. The medical history and clinical profile of the patients in each group are shown in table 2. Whereas groups A and B were similar, the patients requiring transfer to type 4 centers (group C) were younger, had more cardiovascular risk factors, and were more likely to have a history of peripheral arterial disease and a coronary intervention. These patients had a higher frequency of anterior STEMI and AMI-related complications.
Clinical profile by patient group (CC, condition category12)
| Variables | Total (n=4189) | Group A (n=1389) | Group B (n=2627) | Group C (n=173) | P |
|---|---|---|---|---|---|
| Baseline clinical characteristics | |||||
| Age, y | 70 [60-80] | 73 [63-82] | 69 [59-80] | 63 [56-71] | <.001a,b,c |
| Men, % | 68.4 | 66.5 | 68.9 | 75.7 | .032b |
| Active smoking (Z72.0; F17.*), % | 26.9 | 24.0 | 27.2 | 45.1 | <.001a,b,c |
| Dyslipidemia (CC 25), % | 39.1 | 37.7 | 39.4 | 45.1 | .139 |
| Previous PCI (PTCA), % | 13.5 | 12.9 | 12.0 | 41.6 | <.001b,c |
| Previous coronary surgery (CABG), % | 1.34 | 1.44 | 1.33 | 0.58 | .831 |
| Metastatic cancer, acute leukemia, and other severe cancers (CC 8-9)(%) | 1.69 | 1.73 | 1.71 | 1.16 | .977 |
| Diabetes mellitus or complications of diabetes except proliferative retinopathy (CC 17-19, 123), % | 34.8 | 38.9 | 32.2 | 41.0 | <.001a,c |
| Chronic liver disease (CC 27-29), % | 3.82 | 3.38 | 3.96 | 5.20 | .415 |
| Dementia (CC 51-53), % | 4.34 | 4.82 | 4.23 | 2.31 | .276 |
| Major psychiatric disorders (CC 57-59), % | 1.05 | 1.22 | 0.84 | 2.89 | .035c |
| Hemiplegia, paraplegia, or functional deterioration (CC 70-74, 103-104, 189-190), % | 1.00 | 1.01 | 0.91 | 2.31 | .183 |
| Myocardial infarction-related complications (CC 86-87), % | 5.37 | 2.74 | 5.98 | 17.3 | <.001a,b,c |
| Coronary atherosclerosis or angina (CC 88-89), % | 73.2 | 70.5 | 73.2 | 94.8 | <.001b,c |
| Previous valvular heart disease (CC 91), % | 22.6 | 21.0 | 22.2 | 42.2 | <.001b,c |
| Hypertension (CC 95), % | 42.0 | 42.1 | 41.4 | 49.7 | .098c |
| Previous stroke (CC 99-100), % | 1.41 | 1.37 | 1.33 | 2.89 | .238 |
| Cerebrovascular disease (CC 101-102, 105), % | 3.37 | 3.67 | 3.08 | 5.20 | .242 |
| Peripheral vascular disease (CC 106-108), % | 8.50 | 6.91 | 8.95 | 14.5 | .001†‡* |
| Chronic obstructive pulmonary disease (CC 111), % | 8.40 | 9.22 | 7.92 | 9.25 | .34 |
| Pneumonia (CC 114-116), % | 5.59 | 5.47 | 5.29 | 11.0 | .007‡c |
| Chronic kidney disease (CC 135-140), % | 28.7 | 31.7 | 26.5 | 37.0 | <.001a,c |
| Clinical presentation | |||||
| Anterior infarction (I21.01, I21.02, I21.09), % | 48.8 | 46.4 | 49.1 | 64.7 | <.001b,c |
| Myocardial infarction-related complications (CC 86-87), % | 5.37 | 2.74 | 5.98 | 17.3 | <.001a,b,c |
CABG, coronary artery bypass surgery; CC, Condition Category12; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty.
Data are expressed as percentage or median [interquartile range].
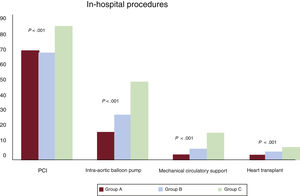
Significant differences in management were seen among the different groups. Patients who required transfer during the hospitalization more frequently underwent revascularization, mechanical ventilation, and circulatory support procedures during the hospitalization, as well as HTx (figure 1, table 3).
Proportion of invasive procedures during hospitalization by patient group. Group A: patients treated during the entire hospital stay in a center with interventional cardiology but without cardiac surgery. Group B: patients treated in hospitals with interventional cardiology and cardiac surgery. Group C: patients transferred to centers with interventional cardiology and cardiac surgery. PCI, percutaneous coronary intervention.
Management and outcomes by patient group
| Variables | Total (n=4189) | Group A (n=1389) | Group B (n=2627) | Group C (n=173) | P |
|---|---|---|---|---|---|
| In-hospital procedures | |||||
| Percutaneous coronary intervention | 69.2 | 69.4 | 67.9 | 85.5 | <.001a,b |
| Coronary revascularization surgery | 2.22 | 0 | 2.97 | 8.67 | <.001a,b,c |
| Intraaortic balloon pump | 23.9 | 15.3 | 26.8 | 48.6 | <.001a,b,c |
| Mechanical circulatory support | 5.3 | 0.6 | 4.12 | 15.0 | <.001a,b,c |
| Mechanical ventilation | 41.3 | 38.2 | 41.5 | 61.8 | <.001a,b,c |
| Extrarenal purification | 2.3 | 1.8 | 2.2 | 8.1 | <.001a,b,c |
| Heart transplant | 1.58 | 0.00 | 2.17 | 5.20 | <.001a,b,c |
| Ventricular assist devices | 0.4 | 0 | 0.4 | 2.9 | <.001a,b,c |
| In-hospital complications | |||||
| Stroke (CC 99-104) | 2.39 | 1.22 | 2.85 | 4.62 | <.001a,b,c |
| Pneumonia (CC 114-115) | 5.85 | 5.40 | 5.79 | 10.4 | .029a,b |
| Acute kidney failure (CC 135) | 12.3 | 8.42 | 13.5 | 26.0 | <.001a,b,c |
| Crude mortality rate, % | 56 | 60.3 | 54.4 | 46.2 | <.001a,b,c |
| Length of hospital stay, d | 7 [2-16] | 8 [2-17] | 15 [8-32] | 5 [1-13] | <.001a,b,c |
| Length of hospital stay for survivors, d | 14 [9-25] | 13 [8-20] | 15 [9-26] | 23 [14-42] | <.001a,b,c |
Data are expressed as percentage or median [interquartile range].
The incidence of in-hospital complications was higher for episodes managed in type 4 centers, particularly in patients who required transfer during the hospitalization (table 3). Similarly, this type of patient had a longer length of hospital stay. Overall, the transferred patients had a lower crude mortality rate (46.2% vs 60.3% in group A and 54.4% in group B; P<.001).
The adjusted model of in-hospital mortality included age and history of revascularization, chronic kidney disease, and renal failure. The discrimination capacity of the model was moderate (AUROC=.71; 95%CI, 0.69-0.72; P<.001), with an acceptable calibration (figure 2 of the supplementary data). The risk-standardized mortality rates were larger in group A (54.8%; 95%CI, 54.5-55.0) than in groups B (53.1%; 95%CI, 52.8-53.3; P<.001) and C (53.6%; 95%CI, 52.6-54.6; P<.001). No significant differences were found in adjusted mortality between groups B and C.
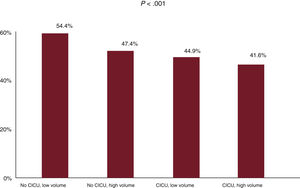
Prognostic impact of structural variables of hospitals in the transferred patient groupThe k-means clustering algorithm identified 57 STEMI-CS episodes during the study period (2016-2020) as a cutoff point. In group C, both high care volume (OR=0.75; 95%CI, 0.64-1.00; P=.009) and CICU availability (OR=0.80; 95%CI, 0.60-0.93; P=.047) showed a significant association with lower in-hospital mortality after the inclusion of the structural variables in the model (figure 2 and figure 3). The interaction between the 2 variables exhibited a significant protective effect (OR=0.65; 95%CI, 0.50-0.85; P=.0014). HTx program availability in centers was not associated with lower in-hospital mortality when the interaction between volume and CICU was considered in the risk adjustment model (OR=0.97; 95%CI, 0.82-1.14; P=0.68). Similarly, after propensity score matching analysis in group C (58 pairs), mortality was lower in patients transferred to high-volume hospitals with CICU availability: average treatment effect of the treated (ATT), 32.8% vs 56.9% (adjusted OR=0.37; 95%CI, 0.16-0.84; P=.009).
Central illustration. Study design, proportion of patients transferred, management, and prognostic impact of structural variables of centers on our patients. CICU, cardiac intensive care unit; MDS, minimum data set; STEMI-CS, ST-segment elevation acute myocardial infarction-related cardiogenic shock.
The main findings of our study are the following: a) in this large series of patients with STEMI-CS treated in an RCC of the SNHS, the percentage of interhospital transfers was low (4.1%); b) the transferred patients were younger, had a worse cardiovascular risk profile, and more frequently underwent revascularization, circulatory support, and HTx during hospitalization; and c) in the group of patients requiring interhospital transfer, those sent to centers with higher volume and CICU availability had lower mortality.
CS-related mortality continues to be unacceptably high, and coronary revascularization in CS associated with ACS is currently the only measure demonstrated to exert a significant prognostic benefit.2 Despite advances in the field of mechanical circulatory support, there is uncertainty about the prognostic impact of this therapeutic approach, which is not yet supported by clinical trial results. The lack of standardized protocols for CS care leads to diagnostic and therapeutic delay and indicates that care is highly dependent on medical team experience, with major differences among centers.15,16 In this regard, the recommendation to organize CS care on a territorial scale by integrating centers of different levels is increasingly widespread. A particularly important consideration is the creation of specialized multidisciplinary teams17 (shock teams) in centers specialized in CS care (shock centers), as well as an organizational model that directs patient transfer from lower- to higher-level centers and those with capacity for advanced treatments. In addition to improving outcomes, this centralization of CS in high-volume centers should help to rationalize health care costs.16
Together with appropriate patient selection, another especially important consideration is the need to minimize unnecessary delays in assessing patients with CS for HTx in order to avoid irreversible multiorgan failure.18 Once a patient is diagnosed with CS at the first medical contact, evidence indicates the value of reporting the case to the closest shock center with HTx capability, ideally within 90 minutes.18 Patients should not be transferred to nonrevascularization-capable centers, and transfer to an RCC can be considered, even if it is not a shock center, if the alternative would involve a transfer time >120 minutes. This necessitates the creation of a very well-organized network allowing early transfer. Taken together, the existence of highly complex interhospital transfer systems is especially important to permit an early and safe response to an interhospital transfer request.
Regardless, there are few data available on the functioning of these circuits in daily clinical practice, the safety of the transfer of patients with this profile, and their outcomes. A previous study in the United States obtained similar findings to our series, with a low percentage of transfers to shock centers (7%)19 and adequate safety. The profiles of the patients transferred in that study are similar to that of our series: both concern younger patients, with greater cardiovascular risk and more frequent history of ischemic heart disease and valvular heart diseases. In that work, patients admitted or transferred to referral hospitals underwent a higher number of procedures and had a longer length of hospital stay, more complications, and lower mortality, similar to our study. In contrast to our series, some of the patients included in the work by Lu et al.19 had CS not associated with STEMI.
Our use of an administrative database did not allow us to categorically state the reasons for the transfers. Regardless, the specific inclusion of CS patients admitted to centers with interventional cardiology capability permits the reasonable exclusion of transfers for urgent coronary angiography, and most transfers were highly likely to have been indicated for advanced treatments. We believe that this hypothesis is supported by the younger profile of the patients in group C, the longer length of hospital stay of patients who survived to admission, and their lower mortality. These data are in line with those of Lu et al.19 The proper selection of patients for advanced treatments is crucial to optimize clinical outcomes in this highly complex context. Increasingly, these decisions are recommended to be made by specialized multidisciplinary teams to avoid futile treatments and optimize patients’ outcomes.
In this setting, a high volume of CS cases has been associated in various studies with improved clinical outcomes.3 Centers treating a higher number of CS patients more frequently apply more complex invasive support procedures.20 Data from both the United States3 and Spain4 show that this distinct approach in high-volume centers results in lower mortality, even after adjustment for early revascularization. One such example is the lower use of pulmonary artery catheters in lower-volume centers. In the study by Lu et al.,19 this approach was more frequently used in patients transferred to high-level centers (26.5% vs 5.7%; P<.01) and was strongly associated with lower mortality (OR=0.63; 95%CI, 0.61-0.65; P<.01). In agreement with these studies, the data from our series clearly show that the transferred patients who were sent to centers with higher care volume had a better clinical course. CICU availability was also a protective factor in our study. Previous data indicate that an adequate staffing of personnel specialized in the management of CS21 is linked to lower incidences of complications and mortality in this setting. In addition, CICU availability in large-scale data of patients with CS in Spain has been associated with a lower adjusted mortality in patients with CS, both due to STEMI4 and other causes.22 The description of better outcomes in patients transferred to other centers with CICU is another contribution of this study. In contrast, the presence of a HTx program was not associated with lower mortality. Notably, centers with HTx availability usually have a higher care volume, CICU availability, and a shock team, factors typically linked to better outcomes in this setting. In addition, transplant centers usually receive high-complexity patients until their potential suitability for advanced treatments is completely defined, which would not be fully detected with the data source used in this work and would lead to worse outcomes. Regardless, the response to that question requires a specific approach with appropriately designed studies.
The scarce information available on the clinical profile and outcomes of patients with CS who require an interhospital transfer is derived from studies conducted in the United States, a country with a very different health care system and geographical distribution from those of Spain. The results of our series provide original data from a large national database on the clinical profile, safety, and outcomes of patients transferred between centers for CS management. As far as we know, this study is the first to analyze this situation in Spain. Along these lines, a recent Spanish expert document23 addressed the challenging implementation of the shock code with multidisciplinary and centralized care in experienced high-volume centers, with the aim of minimizing the inequity in the management of these patients. In this regard, our study data could be very useful for tackling this demanding organizational challenge.
The present study has several limitations, including those inherent to the use of an administrative database that lacks variables of interest, such as hemodynamic profile and transfer reasons and times. Nonetheless, the use of this type of database has been sufficiently validated for the prediction of clinical events vs clinical registries.24 The retrospective and observational nature of the study means that we cannot rule out a certain degree of selection bias and residual confounding. Finally, the availability of data on shock team presence in the centers would have enabled analysis of the contribution of this major factor.5,6 Despite these limitations, our study provides novel and highly useful data on the clinical profile and outcomes of patients with CS who undergo interhospital transfer in daily clinical practice in Spain. This information may be crucial for the appropriate regional organization of CS management in Spain.
CONCLUSIONSA low percentage (4.1%) of patients with STEMI-CS treated in RCCs undergoes interhospital transfer in Spain. The transferred patients are younger, have a worse cardiovascular risk profile, and more frequently undergo invasive procedures during the hospitalization. Of the transferred patients, those sent to centers with CICU availability with a high care volume have a better clinical course.
- -
Patients with CS have considerable morbidity and mortality and health care resource utilization.
- -
Clinical practice guidelines recommend the centralized management of these patients in high-level centers with 24-hour availability of interventional cardiology rooms and mechanical circulatory support devices.
- -
Implementation of the shock code represents an organizational challenge that requires facilitation of interhospital transfer to referral centers and systems.
- -
A low percentage of patients with CS and managed in RCCs undergo interhospital transfer in Spain.
- -
The transferred patients are younger, have a worse risk profile, and more frequently undergo invasive procedures during the hospitalization.
- -
Of the transferred patients, those sent to high-volume centers with cardiac intensive care units have lower mortality.
This work was conducted via an agreement between the Institute for Healthcare Improvement (IMAS Foundation) and the Spanish Society of Cardiology.
AUTHORS’ CONTRIBUTIONSM. Barrionuevo-Sánchez, A. Ariza-Solé, and F.J. Elola contributed to the study design, data analysis and interpretation, and manuscript drafting. N. del Prado, N. Rosillo, J.L. Bernal, and C. Fernández-Pérez contributed to the data analysis and interpretation. A. Viana-Tejedor, P. Jorge-Pérez, J.C. Sánchez-Salado, V. Lorente, O. Alegre, I. Llaó, R. Martín-Asenjo, J. Pascual, M. Corbí-Pascual, M. Marcos, F. de la Cuerda, J. Carmona, and J. Comin-Colet contributed to the data interpretation and manuscript supervision.
CONFLICTS OF INTERESTNone.
We thank the Spanish Ministry of Health for the help provided to develop the RECALCAR project, with special thanks to the Institute for Health Information.