Hypertrophic cardiomyopathy (HCM) is a primary myocardial disease that is typically transmitted in an autosomal dominant fashion. HCM is characterized by broad heterogeneity in clinical expression. Although conduction disturbance is described in HCM,1 the occurrence of high-grade atrioventricular block is unusual in children and represents a clinical decision-making challenge. Here, we report a 12-year-old girl, with HCM and a subcutaneous implantable cardioverter-defibrillator (s-ICD) for a previous aborted cardiac arrest, who subsequently developed syncopal paroxysmal atrioventricular block.
The patient was initially referred to our hospital at the age of 8 years, after a ventricular fibrillation episode that was successfully defibrillated. HCM with left ventricular noncompaction and restrictive physiology had been previously diagnosed at another institution. Septal wall myocardium was mildly thickened, and systolic function was normal; there was no left ventricular outflow tract obstruction and both atria were severely enlarged. Cardiac catheterization showed an increased left ventricular filling pressure. The patient underwent s-ICD implantation and, after a 4-year follow-up, she has not received any appropriate therapy, under no medical treatment.
There was no family history of HCM or sudden cardiac death. The parents, from Morocco, were first-degree cousins. A genetic test by next-generation sequencing identified the homozygous pathogenic variant Asp778Glu in MHY7 in the proband.
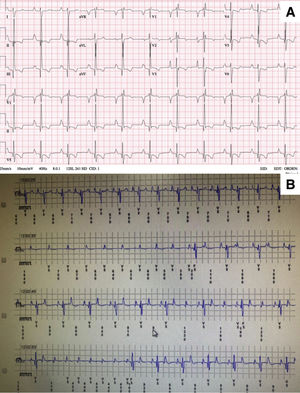
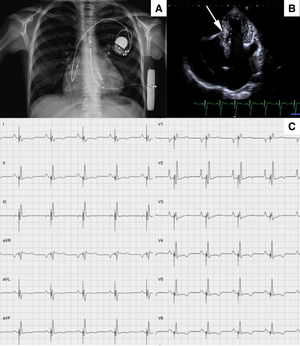
At the age of 12 years, the patient developed unexplained syncopal attacks, with no arrhythmia recorded in the defibrillator. The resting electrocardiogram remained unchanged (figure 1A), so a subcutaneous loop recorder was implanted, demonstrating an intermittent high-degree atrioventricular block that coincided with the loss of consciousness (figure 1B). A definitive dual chamber endovascular pacemaker implant was decided; through the axillary vein, a lumenless 4-Fr lead was used (Select-Secure model 3830 69cm, Medtronic Inc., United States) (figure 2A,B) through a fixed preformed sheath (C315 HIS, Medtronic Inc, United States) connected to the digital recording system (Electrophysiology Lab System, United States) in a unipolar configuration for recording the intracavitary signal. After locating the His electrogram, the sheath was advanced 1 to 2cm in the apical direction; once there, when a paced QRS with “W” morphology was obtained in lead V1, the electrode was inserted into the septum with 5 to 6 clockwise turns until the notch in the paced QRS complex migrated toward the end of the QRS wave and the QRS width narrowed. No contrast was injected through the sheath. Excellent pacing parameters (R sensed wave 12mV with a capture threshold 0.5 Volts at 0.4ms of impulse width) were obtained; subsequently a 52cm lead was implanted in the right atrial appendage (figure 2A,B). Total fluoroscopy time was 8minutes. The pacemaker was finally programmed in AAI-DDD mode. Given the narrow QRS complex with extreme similarity to the patient's resting electrocardiogram (figure 2C), intraoperative s-ICD electrocardiogram screening showed no failure to identify the paced QRS (3 vectors passed), confirmed 24hours later. The girl has remained well after 6 months of follow-up, with no syncopal recurrences in New York Heart Association functional class II, no additional malignant ventricular arrhythmias, and optimal pacemaker parameters (R sensed wave 15mV with a capture threshold 0.75 Volts at 0.4ms). The ventricular pacing rate was 7%.
Our report describes a child with an aggressive form of HCM with restrictive physiology due to a homozygous mutation in MHY7. The main novel therapeutic approach we provide is the coexistence of an s-ICD together with endovenous left bundle branch pacing to resolve a complex clinical situation. In our patient, when we decided to implant the ICD for secondary sudden cardiac death prevention, no pacing or antitachycardia treatment was expected to be required; hence, to overcome the risks of intravascular lead failure in a young and growing patient, we selected an s-ICD. Nevertheless, when symptomatic atrioventricular block made pacing unavoidable, the left bundle branch pacing option seemed to offer several potential benefits over a single endovenous ICD system: first, the high rate of adverse events with endovascular ICD generator and leads is well known, especially in young patients2; in addition, left bundle pacing is an emerging technique to deliver a more physiological pattern of ventricular pacing, generating a narrow QRS complex and promoting atrioventricular and intraventricular synchrony, thus avoiding adverse consequences of right ventricle pacing on left ventricular function and with lower thresholds and higher R detection than His bundle pacing.3 Finally, a similar paced QRS to the resting QRS allows correct working of the s-ICD, avoiding a failing in electrocardiogram screening and, thus, reducing the chance of inappropriate therapies.
A challenging situation will appear when the s-ICD battery runs out. One option would be to remove the s-ICD system and insert a transvenous defibrillation electrode with a cardiac resynchronization therapy defibrillator device, as long as the girl's size is close to that of the adult. This would allow us to have only 1 generator placed, enhancing the aesthetic result, as well as to dispose of antitachycardia pacing therapy. However, it would mean another element of interference with the tricuspid valve in a young person.
In summary, the combined use of an s-ICD with a left bundle branch pacemaker may be the optimal choice in certain situations, especially in children, in whom deleterious effects of chronic pacing, as well adverse events related to endovenous defibrillator leads, are extremely undesirable.
CONFLICTS OF INTERESTM. Álvarez reports personal fees from Boston Scientific and Medtronic, outside the submitted work. The other authors have nothing to disclose.