The European Society of Cardiology (ESC) recently presented their guidelines for the management of chronic coronary syndromes (CCS), updating the 2019 guidelines with new data and recommendations.1,2 In this review, performed by a group of experts at the suggestion of the Spanish Society of Cardiology (SEC) Guidelines Committee, we summarize the most significant additions, discuss the implications of their implementation in our setting, and identify gaps in evidence.
WHAT IS NEW?The guidelines define CCS as a range of clinical presentations resulting from structural and/or functional alterations related to chronic diseases of the coronary arteries and/or microcirculation. The clinical spectrum of CCS includes the following entities: stable obstructive coronary artery disease (CAD) after acute coronary syndrome, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG); stress-induced angina, or equivalent, with obstructive CAD; angina with no obstructive CAD (ANOCA) or ischemia with no obstructive CAD (INOCA); asymptomatic patients with abnormal coronary anatomical or functional test results; and left ventricular (LV) dysfunction or heart failure of ischemic origin.
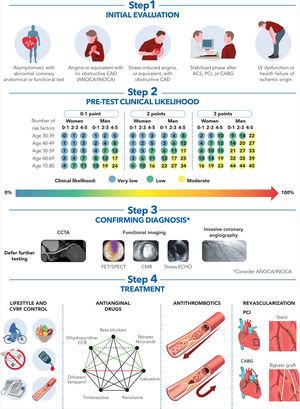
DiagnosisThe new guidelines introduce a streamlined 4-step sequential clinical approach for patients with suspected CCS, replacing the previous 6-step protocol:
Stepwise 1This step includes a general clinical evaluation focused on assessing symptoms—where “typical” vs “atypical” angina is replaced by a detailed description of symptoms—and signs of CCS, 12-lead resting electrocardiogram (ECG), basic blood tests, and in selected individuals, chest X-ray and pulmonary function testing.3 High-sensitivity C-reactive protein and/or fibrinogen plasma levels should be considered (IIaB).
Stepwise 2This step involves echocardiography and a new estimation of the pretest clinical likelihood of obstructive CAD to assess the need for further testing by using the risk-factor-weighted clinical likelihood (RF-CL) model, which takes into account age, sex, angina symptoms, and the number of risk factors (IB). If the pretest likelihood of obstructive CAD is very low (< 5%), deferral of further diagnostic tests should be considered. For a low (5%-15%) pretest likelihood of obstructive CAD, a coronary artery calcium score should be considered to reclassify patients and identify more individuals with a very low (≤5%) coronary artery calcium score-weighted clinical likelihood (IIaB). Exercise ECG testing may also be considered an alternative test to rule in or rule out CAD when noninvasive imaging tests are unavailable. It is also recommended in selected patients to assess exercise tolerance, symptoms, arrhythmias, blood pressure response, and event risk (IC)
Stepwise 3This step includes diagnostic testing to establish the diagnosis of CCS and determine patients’ risk of future events. Coronary computed tomography angiography (CCTA) is established as a first-line diagnostic test4: in individuals with suspected CCS and low to moderate (> 5%-50%) pretest likelihood, it is recommended to diagnose obstructive CAD and estimate the risk of events (IA). CCTA features such as low attenuation plaque, positive remodeling, spotty calcifications, and the napkin-ring sign are associated with an increased risk of death or nonfatal myocardial infarction. Functional imaging tests, including stress echocardiography, myocardial perfusion scintigraphy (SPECT and PET), and stress cardiac magnetic resonance (CMR) imaging, are recommended in individuals with suspected CCS and moderate-to-high (> 15%-85%) pretest likelihood of obstructive CAD to estimate the risk of future events. Invasive coronary angiography is recommended to diagnose obstructive CAD in individuals with a very high (> 85%) clinical likelihood of disease, severe symptoms that are refractory to guideline-directed medical therapy, angina at low levels of exercise, and/or high event risk. Additionally, the availability of coronary pressure assessment is advised, and it should be used to evaluate the functional severity of intermediate, nonleft main stenoses before revascularization (IA). For intermediate stenoses, fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR) remain standard techniques, with significance thresholds of ≤0.8 or ≤0.89, respectively (IA).
Stepwise 4This step focuses on lifestyle and risk-factor modification, as well as the use of disease-modifying therapy, including medications or revascularization as needed.
TreatmentA patient-centered approach is recommended to address treatment goals, including patient-reported outcome measures and patient-reported experience measures. Health outcomes improve with effective communication and increased patient involvement, in which shared decision-making is central to future patient care and long-term follow-up. Controlling risk factors is essential for preventing disease progression and enhancing prognosis in patients with CCS. Multidisciplinary interventions that address risk factors and symptom management are increasingly associated with improvements in self-care and lifestyle modifications.
The section on antianginal medications has been shortened, as most information has been relocated to the supplemental data section. These guidelines have removed the classification of antianginal medications into first- and second-line treatments, recommending that physicians choose therapies based on each patient's hemodynamic profile, comorbidities, concomitant medications, and the underlying pathophysiology of myocardial ischemia. However, the authors acknowledge that treatment should be started with a beta-blocker or a calcium channel blocker. To assist physicians in selecting the most suitable antianginal drug and in determining combinations, the authors have included a helpful diamond (figure 1). Lastly, the authors have issued a class III indication (not recommended) for the use of ivabradine as add-on therapy in patients with CCS, a left ventricular ejection fraction (LVEF) >40%, and no clinical heart failure.
Central illustration. Management of patients with chronic coronary syndromes.
ACS, acute coronary syndrome; ANOCA, angina with no coronary artery disease; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CCB, calcium channel blocker; CMR, cardiac magnetic resonance; CVRF, cardiovascular risk factors; ECHO, echocardiography; INOCA, ischemia with no coronary artery disease; LV, left ventricle; PET, positron emission tomography; PCI, percutaneous coronary intervention; SPECT, single photon emission computed tomography.
Regarding event prevention, the longest section is dedicated to antithrombotic therapy. New evidence indicating that clopidogrel has better efficacy and a lower or similar bleeding risk compared to aspirin has prompted the task force to recommend clopidogrel monotherapy as a safe and effective alternative to aspirin for long-term secondary prevention. Another updated recommendation is for lifelong aspirin treatment in patients without a history of myocardial infarction or revascularization but with evidence of significant obstructive CAD.
The duration of dual antiplatelet therapy following stenting is guided by bleeding and thrombotic risks, with the default recommendation being six months. In patients at high ischemic risk without a high bleeding risk, prolonged dual antiplatelet therapy with aspirin plus either ticagrelor 60mg twice daily (bid), low-dose rivaroxaban, clopidogrel, or prasugrel is equally recommended. The option of ticagrelor 90mg bid monotherapy is also introduced (IIbC).
In terms of lipid-lowering drugs, the guidelines introduce bempedoic acid as an effective treatment for patients intolerant to statins (IB) or as an add-on therapy in those not achieving their goals with statins and ezetimibe (IIaC). Interestingly, the guidelines recommend the use of semaglutide in patients with CCS who are overweight (body mass index >27kg/m2) or obese to reduce cardiovascular events. Additionally, colchicine (0.5mg/d) is recommended to reduce the risk of myocardial infarction, stroke, and the need for revascularization in patients with CAD (IIaA).
The guidelines include several significant updates regarding myocardial revascularization. Prior to considering any invasive strategy, optimizing medical therapy through guideline-directed medical therapy (GDMT) is strongly encouraged. This is especially relevant in light of recent evidence for patients with heart failure (HF) and CAD. Despite initial controversy surrounding the benefits of revascularization following the publication of the ISCHEMIA trial results,5 further analysis of long-term outcomes, meta-analyses, and other clinical trials have supported the role of revascularization in improving survival in patients with extensive coronary artery disease, such as 3-vessel disease (IA), left main CAD (IA), and severe stenosis of 1 or 2 vessels, including the left anterior descending artery (IB).
A key aspect of the new guidelines is the personalized selection of the revascularization strategy. The authors emphasize the need for an individualized approach when deciding between PCI and CABG. Key factors to consider include the extent and complexity of CAD, which can be quantified using the SYNTAX score (IB); the presence of comorbidities, particularly diabetes; the assessment of surgical risk with the Society of Thoracic Surgeons (STS) score (IB), as opposed to EuroSCORE or other scales; ventricular function; and patient preference (IC). If PCI is selected to treat complex lesions—such as left main lesions, bifurcations, or long lesions—the authors recommend the use of intracoronary imaging guidance via intravascular ultrasound or optical coherence tomography (OCT) (IA). As in previous guidelines, it is advised that therapeutic decisions be made by a multidisciplinary team, or Heart Team, to ensure the most appropriate strategy for each individual case (IC).
The new guidelines provide more clinically oriented and practical recommendations on the best revascularization strategy compared with medical therapy alone in different scenarios:
Ventricular dysfunctionThere is an emphasis on team-based decision-making for patients with LEVF ≤35% and multivessel disease. When surgical risk is acceptable, CABG is the preferred option (IB) over medical therapy to improve survival. PCI may be an alternative in patients with high surgical risk (IIbB).
Multivessel disease with diabetesThe guidelines recommend CABG to improve survival (IA). However, in patients with very high surgical risk, PCI with newer-generation drug-eluting stents is considered an acceptable alternative (IIaB).
Three-vessel disease without diabetesCABG is indicated to improve survival, health outcomes, and alleviate angina (IA). while PCI is recommended due to its lower invasiveness and generally noninferior survival rates, particularly in patients with low-to-intermediate anatomic complexity, in whom PCI can achieve a level of revascularization comparable to that of CABG (IA).
Severe left main coronary artery diseaseCABG is recommended over medical therapy to improve survival, regardless of the complexity of coronary anatomy (IA). PCI is recommended in patients with a low SYNTAX score, provided that complete revascularization similar to that achieved with CABG is attained (IA). PCI may also be considered (IIaA) in patients with an intermediate SYNTAX score.
Chronic coronary syndromes and heart failureAbout half of HF patients have ischemic etiology, with an increasing proportion of ischemic HF with preserved EF (HFpEF) seen in the last years, with a relevant role of microvascular angina due to coronary microvascular disfunction. The evaluation of inducible ischemia is important in patients with HF, and as clinical assessment alone may underestimate the presence of CAD, functional imaging, especially in HFpEF patients, should be considered to detect potential benefits from revascularization. CCTA is also recommended in patients with a low-to-intermediate pretest likelihood of obstructive CAD, and those with equivocal noninvasive stress tests. In HF patients with LVEF ≤35% in whom obstructive CAD is suspected, direct invasive coronary angiography is recommended (IB). In selected patients with HF with reduced EF (HFrEF) undergoing high-risk PCI, the use of a microaxial flow pump may be considered (IIbC).
Angina and ischemia with no obstructive coronary artery diseaseIn patients with refractory angina that significantly impacts quality of life and suspected or confirmed ANOCA/INOCA (ie, anginal symptoms with normal coronary arteries, nonobstructive lesions on noninvasive imaging, or intermediate stenoses with normal FFR/iFR during coronary angiography), invasive coronary functional testing is recommended to define the underlying endotypes and guide appropriate treatment, considering patient preferences (IB). In symptomatic patients with ANOCA/INOCA, medical therapy tailored to the results of coronary functional tests should be considered to improve symptoms and quality of life (IIaA). In addition, in individuals with suspected vasospastic angina and frequent symptoms, ambulatory ST-segment monitoring should be considered to detect ST-segment deviations during anginal episodes (IIaB).
Health outcomes improve with proper communication and greater patient involvement, in which shared decision-making is central to future patient care and long-term follow-up. This long-term follow-up, in patients with CCS who have established CAD (prior acute myocardial infarction, revascularization, or known CAD—or nonobstructive CAD), includes monitoring for disease progression. Risk stratification is also recommended in patients with new or worsening symptoms, preferably using stress imaging.
CONSEQUENCES OF IMPLEMENTING THE GUIDELINES IN OUR SETTINGDiagnosisDetailed medical history and physical exams require primary care physicians to dedicate time to patient care. Current recommendations, upgrading the use of CCTA as a first-line test, conflict with its underuse in our setting, mainly due to limited availability. Similarly, stress CMR and other functional tests, such as PET, also face significant availability issues, which hampers the proper implementation of diagnostic recommendations. The guidelines emphasize the need for both appropriate technology and professional training to ensure standard care. As a well-trained imaging team is a prerequisite for CCTA, it is essential to focus our efforts on providing professionals with specific training in cardiac imaging and standardizing the acquisition and reporting of these studies.
The new guidelines mark a significant advancement in risk stratification for CAD by introducing an algorithm based on symptoms and risk factors. This algorithm highlights the importance of complementary tests such as ECG, noninvasive functional assessment, and CCTA in patients with moderate-to-high risk profiles. Following these recommendations, there should be a substantial increase in the use of CCTA, which currently shows significant variability in implementation among different regions and health care centers in the country.6
Regarding invasive testing, the guidelines clearly establish the indication for pressure wire assessment of intermediate stenoses (40%-90% diameter stenosis for nonleft main stenosis and 40%-70% diameter stenoses for left main lesions). This is particularly important, as the degree of reclassification of the significance of stenosis after pressure wire assessment is highly notable in our setting.7 Although the use of pressure wire has steadily increased in recent years, its use still lags behind that of neighboring countries.6
TreatmentAn increasing number of centers in Spain are implementing multidisciplinary teams to assess patients comprehensively and involve them in therapeutic decisions. However, there is no single effective model, and limited evidence exists evaluating the efficiency and outcomes for patients. The uneven implementation of educational programs in patients with CAD could be improved through tele-rehabilitation, which is an effective alternative to reach a larger number of patients. Furthermore, the Spanish health care system does not refund GLP-1 medications for weight loss in nondiabetic patients, and there are restrictions on smoking cessation drugs, hindering their widespread availability to all patients. Additionally, the lack of commercialization of some recommended treatments, such as nicorandil or low-dose rivaroxaban, and the absence of an approved indication for the use of colchicine in CCS patients are further limitations to the implementation of these guidelines in our setting.
Implementing these new clinical guidelines is crucial, considering certain regional factors specific to Spain. The guidelines recommend decision-making based on Heart Teams, focusing on patient health outcomes, and align with the Cardiovascular Health Strategy of the Spanish Ministry of Health,8 facilitating their deployment and implementation. Spain continues to have one of the lowest rates of revascularization (PCI or CABG).9 The recommendations outlined in these guidelines should be promoted beyond the field of cardiology, involving the health administration to overcome barriers to patient access to these invasive therapies.
Another significant aspect of the guidelines is the evaluation of patients with ANOCA/INOCA. The guidelines emphasize the need for invasive assessment when these conditions are suspected, aiming to identify the patient's phenotype (endothelial dysfunction, epicardial spasm, microvascular spasm, or microvascular dysfunction) to guide targeted pharmacological treatment or to exclude a coronary cause of the symptoms. Given the high prevalence of these conditions, a considerable increase in the number of invasive studies in this area is expected.6
Despite robust evidence of benefits in terms of mortality and morbidity, adherence to guideline-directed medications remains suboptimal. Behavioral and mobile health interventions, including apps and wearable devices, are recommended to improve patient adherence to healthy lifestyles and medical therapy during long-term follow-up. Simplifying medication regimens (eg, using fixed-dose drug combinations) along with multiprofessional and family involvement is also recommended to enhance patient adherence to medications and promote patient education and involvement.
GAPSIncreased standardization in reporting CCTA to emphasize key plaque features is necessary to systematically gather prognostic information and enhance risk management strategies. Quantitative stress perfusion CMR is a promising tool, particularly for assessing microvascular dysfunction through myocardial blood flow quantification. However, it still requires further standardization and experience for effective application in clinical practice. FFR-CT can supplement CCTA by providing model-based computational FFR values, but it remains a costly technique with limited availability and certain drawbacks.
Residual ischemia following PCI, as determined by FFR/iFR, may indicate the presence of residual atherosclerotic lesions, suboptimal PCI results, or ongoing microvascular dysfunction. However, it is uncertain whether post-PCI FFR/iFR is a truly modifiable risk factor, and additional evidence is needed to clarify its role in post-PCI management and long-term outcomes.
Educational programs targeting modifiable risk factors have proven effective in improving diet, knowledge, and physical activity, with longer programs (≥3 months) generally yielding better results. The necessity of repeating programs to maintain adherence to healthy lifestyles remains unclear. Regarding medical therapy, a significant gap in the evidence exists due to the lack of prognostic studies on most antianginal treatments, such as nitrates, calcium channel blockers, and beta-blockers (with the exception of HF or postmyocardial infarction cases). Another important gap is the use of aspirin in asymptomatic patients with nonobstructive CAD diagnosed via CCTA. Considering the SECURE trial results, the potential of the polypill as a new secondary prevention strategy aimed at reducing cardiovascular events is overlooked.10 Similarly, the absence of a recommendation for icosapent-ethyl as a treatment to reduce cardiovascular events in CCS patients is concerning.
Regarding revascularization, significant limitations and knowledge gaps persist, warranting further study for future guidelines. These include evaluating the impact of GDMT vs GDMT plus an invasive approach on all-cause mortality in patients with CCS, clarifying how revascularization affects cardiovascular and noncardiovascular mortality, defining the concept of incomplete revascularization (anatomical vs functional) and its long-term effects, determining whether PCI and CABG are comparable in patients with HFrEF (especially in light of new HF treatments), and investigating the safety and efficacy of hybrid revascularization using minimally invasive surgery for the left anterior descending artery combined with PCI.
The pharmacological management of ANOCA/INOCA remains largely empirical, highlighting a significant gap in the current guidelines. There is a need for prospective, randomized clinical trials to evaluate the efficacy of antianginal therapies in improving symptoms and clinical outcomes among the various endotypes of these conditions. Additionally, research on effective methods to support healthy lifestyle behaviors—including the implementation of health-promoting policies and practices in workplace settings—and sustain medication and healthy lifestyle adherence over time is still needed.
CONCLUSIONSThe new guidelines for the management of CCS offer clinically oriented and practical recommendations for developing diagnostic algorithms and therapeutic guidance. This commentary aims to summarize these novelties, highlight the challenges of their implementation in the Spanish clinical setting, and identify the remaining gaps in evidence.
FUNDINGThis article has not received any funding.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
SEC Guidelines Committee: Pablo Avanzas (president), Pilar Mazón, (secretary), Rut Andrea Ribas, Marisol Bravo Amaro, Alberto Cordero Fort, Marisa Crespo, F. Javier Jiménez Candil, María Antonia Martínez Momblan, Sonia Mirabet Pérez, Juan Sanchis Forés, Marta Sitges Carreño, José M. de la Torre, Javier Torres Llergo, and David Vivas.
SEC Working Group for the 2024 ESC Guidelines for the management of chronic coronary syndromes: David Vivas (coordinator), Belen Cid (coordinator), Manuel Carnero-Alcázar, Juan Cosín-Sales, Elena Díaz-Peláez, Silvia Pérez-Ortega, Nieves Romero, and Oriol Rodríguez-Leor.
The names of all the authors of the article are listed in alphabetical order in Appendix A.
SEE RELATED CONTENT:.
https://secardiologia.es/cientifico/guias-clinicas/cardiopatia-isquemica/15236-2024-esc-guidelines-for-the-management-of-chronic-coronary-syndromes.
Corresponding author.
E-mail address:dvivas@secardiologia.es (D. Vivas).