Keywords
INTRODUCTION
The definitive treatment of patients with atrial fibrillation (AF) includes isolation of the potential triggering arrhythmogenic foci located predominantly in the pulmonary veins (PV),1 as well as the abolition of nonpulmonary foci,2,3 employing radiofrequancy (RF) as the source of energy. Individuals with paroxysmal atrial fibrillation (PAF) constitute the subgroup that most benefits in terms of efficacy, in comparison with chronic forms.4
The typically reported complications of RF include PV stenosis,5 atrioesophageal fistula,6 thromboembolism,7 and postablation atrial arrhythmias.8
The lesion produced by cold, in contrast to that obtained by means of RF, preserves the tissue architecture and reduces the formation of thrombi.9
Cryoablation using a catheter or a balloon catheter has been found to be safe in animals and humans as it enables the complete circumferential isolation of the veno-atrial junction in the pulmonary vein antrum.9,10
In this report, we present our initial experience with balloon catheter cryoablation, focusing on complete, acute circumferential isolation of the PV in patients with PAF; to the best of our knowledge, this is the first observation in Spain with this technique.
METHODS
Device
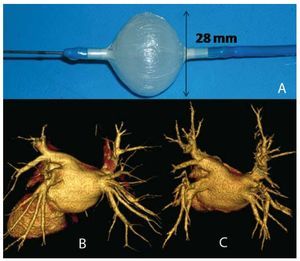
We used the 28-mm, 10.5 French (F) Artic Front double-lumen balloon catheter (CryoCath Technologies, Montreal, Quebec, Canada) (Figure 1) that allows the circulation of nitrous oxide at temperatures of -30ºC to -75ºC; the cooling vapor absorbs the heat of the surrounding tissue, thus resulting in its freezing. After each application, the gas is evacuated to the exterior of the system.
Figure 1. A: inflated balloon catheter. B: computed tomography (64 slices) of the pulmonary veins, case no. 5. C: computed tomography (64 slices) of the pulmonary veins, 3 months postablation, case no. 5.
The cooling vapor is released by means of a console equipped with a monitor that provides data on the temperature reached and the duration of the application.
Patients
Five patients were included (4 men and 1 woman) with a mean age of 58.6 years (range, 53-72 years), with no structural heart disease and a documented history of 2 to 6 years of recurrent PAF refractory to antiarrhythmic therapy. The left ventricular ejection fraction (LVEF) was 58% or greater in every case (Table 1).
Studies Prior to the Procedure
All the patients underwent transthoracic and transesophageal echocardiogram at least 48 hours prior to the procedure and 64-slice computed tomography angiography (Figure 1) in order to define the anatomy of the left atrium and the PV (Table 2).
Procedure
The procedures were carried out with general anesthesia; intubation was achieved using cisatracurium as the neuromuscular blocker.
A 6-F decapolar electro catheter was introduced up to the coronary sinus and a 6-F quadripolar catheter up to the veno-atrial junction for the proximal bipolar recording of the His bundle potential.
Following transseptal puncture, anticoagulation was maintained with an activated coagulation time (ACT) longer than 300 ms. Once the SL0 or SL1 sheath was introduced into the left atrium, a St. Jude 404878 260-cm 0.32 guidewire with J tip was passed selectively into each of the PV, and phlebography was performed with injection of 50% contrast medium. After angiography, the 15-F Flex-Cath guide catheter was passed through the guidewire with its dilator (CryoCath Technologies), and was positioned with the distal portion in the atrial cavity, in 40º left anterior oblique projection, and the 7-F dodecapolar circular catheter with adjustable diameter (St. Jude Reflexion Spiral) was introduced for cartography; each and every PV was mapped, starting systematically with left superior PV, followed by left inferior, right superior and right inferior PV.
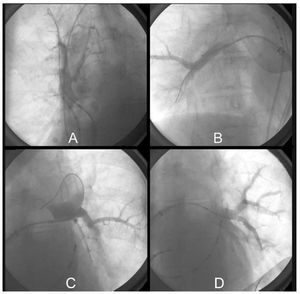
After mapping, each of the veins was catheterized selectively with the 28 mm diameter balloon catheter, which was adjusted to the antrum until occlusion was achieved, with retention of 50% contrast medium in the interior of the vein and absence of drainage into the atrial cavity (Figure 2). Once this was confirmed, the lumen of the catheter was rinsed with 3 to 5 mL of heparinized saline solution in order to prevent crystallization of the contrast medium due to the cold, and the freezing process was initiated, keeping pressure of the balloon against the vein for 90 seconds; after this time, the balloon was completely adhered to the PV antrum, and the freezing process was continued for a total of 300 seconds.
Figure 2. Ablation. Balloon catheter occlusion showing retention of the contrast medium with absence of atrial drainage due to complete occlusion, case no. 5. A: balloon catheter occlusion of right superior pulmonary vein. Note the quadripolar catheter in superior vena cava for phrenic nerve stimulation. B: balloon catheter occlusion of right inferior pulmonary vein. C: balloon catheter occlusion of left superior pulmonary vein. D: balloon catheter occlusion of left inferior pulmonary vein.
Cold was applied between 2 and 3 times to each vein (mean, 2.75 times) individually, and the quadripolar Hisian catheter was introduced up to the superior vena cava for continuous phrenic nerve stimulation at low frequencies (3000 ms of cycle length), for the purpose of monitoring its integrity during cryoablation of the right PV, especially the superior veins.
Once the procedure was over, and after the sheaths had been removed from the transseptal catheter anticoagulation was reversed using protamine and low-molecular-weight heparin was introduced. Four hours later, a loading dose of oral dicumarol and 300 mg of acetylsalicylic acid (ASA) was initiated.
RESULTS
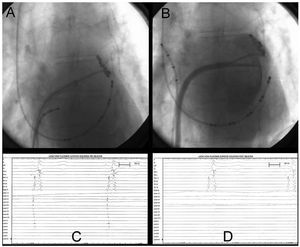
Thirty minutes after the applications of cold had been completed, the PV were again mapped with the circular catheter, and their complete isolation (100%) was demonstrated (Figures 3C and 3D), with mapping of the antrum anterior to the ostium and inside the PV itself (Figures 3A and 3B). The entry and exit blocks were demonstrated with stimulation.
Figure 3.Case no. 5. A: catheter mapping in antrum of left superior pulmonary vein prior to ablation. B: catheter mapping in antrum of left superior pulmonary vein with injection of contrast medium following ablation. C: intracavitary recording prior to ablation obtained in Figure 3A with the circular catheter in the outer portion of the pulmonary vein ostium, with arrhythmogenic potentials of the pulmonary veins (PVAP). D: recording obtained in the same position as in Figure 3A following ablation, with absence of PVAP, showing complete circumferential isolation.
The mean temperature reached in all the applications was higher than -40ºC (range, -32ºC to -70ºC).
The mean total duration of the procedure was 5.4 hours (329 minutes), with a mean fluoroscopy time of 81.6 minutes.
Course
The patients spent the first 24 hours post-procedure in the intensive care unit and were discharged from the hospital within 48 to 72 hours, with no complications, and with 300 mg flecainide, ASA and oral anticoagulation for the first three months post-ablation.
Three months post-ablation, non stenosis of the PV and non recurrence of arrythmia were documented, with follow-up involving Holter at 7, 15, 30, and 90 days and multislice computed tomography at 30 and 90 days.
DISCUSSION
Radiofrequency ablation in the area of the PV orifice is the most widely utilized method for the definitive treatment of AF.
This method, in all of its technical variants, requires technical skill and experience, since it necessitates prolonged procedures with considerable fluoroscopy times.
Moreover, RF applications in the ostium can produce the stenosis of one or more PV5 and, due to the close anatomical relationship,11 more lethal complications such as atrioesophageal fistula.6
The initial experiences reported in humans, involving the application of cold using a given catheter, suggest that cryoablation may be safer than RF ablation since it can reduce the risk of PV stenosis and other complications, such as atrioesophageal fistula, and achieves immediate acute isolation of 97% of the PV.12
The use of the novel Arctic Front balloon catheter10 has been associated with the absence of PV non stenosis and absence of atrioesophageal fistula.
In short, we present here the immediate acute results for the first cases done in Spain with the cryoballoon catheter as a faster and safer method to achieve complete acute circumferential isolation of the arrhythmogenic focus in the PV in patients with PAF.
A greater number of patients, with medium-term and long-term follow-up, and comparative studies involving other methodologies will ultimately enable us to define the role of cryoablation in the curative treatment of PAF.
ACKNOWLEDGMENTS
We wish to express our thanks to Dr Juan J. Fernández-Ramos, medical director of our hospital, for his continued support in health care and research and to Gema Mariscal for preparing the manuscript.
Declaration of conflict of interests: Dr Robert H. Hoyt is a USA researcher for the CryoCath system.
Correspondence: Dr. J.M. Paylos.
Laboratorio de Electrofisiología. Unidad de Arritmias. Clínica Moncloa. Avda. de Valladolid, 83. 28008 Madrid. España.
E-mail: jpaylos@ircus.com
Received January 14, 2009.
Accepted for publication April 17, 2009.