Keywords
INTRODUCTION
Coronary artery disease is associated with a less favorable outcome in diabetic than in non-diabetic patients.1 Several factors, including poor development of new collateral circulation after a coronary occlusion may contribute to this increased morbidity and mortality.2,3
The neovascularization process in adults has been determined to result from angiogenesis (new vessels formed from old vessels) combined with vasculogenesis (new vessels formed from precursor cells).4 Stem cell transplantation has shown promise as a new therapeutic approach with the potential for inducing neovascularization in "no-option" patients with chronic ischemia.5 Several small clinical studies have described the beneficial effect of transendocardial injection of autologous bone marrow mononuclear cells (BMMNCs) in patients with end-stage, chronic ischemic heart disease.5-7
It has been well demonstrated that the angiogenic process is impaired in patients with diabetes.8,9 In vitro studies have suggested that endothelial progenitor cells (EPCs), may be functionally defective in diabetics. However, whether the clinical effect of cell treatment is comparable between diabetics and non-diabetic patients remains unknown.
Therefore, we analyzed a cohort of end-stage heart failure patients to compare the clinical and functional data following cell transplantation in diabetics versus nondiabetic patients.
METHODS
Patient Population
From December 2001 to May 2006, 26 consecutive patients with end-stage ischemic heart disease, and no-option for standard coronary revascularization were treated with BMMNCs as part of 2 clinical trials led by the Texas Heart Institute (Houston, TX, USA). The first trial was performed in collaboration with Pro-cardiaco Hospital (Rio de Janeiro, Brazil), and the second was performed at St. Luke's Episcopal Hospital (Houston, TX). The inclusion and exclusion criteria were similar in both phase I studies and have been described elsewhere.5 In brief, patients were included if they had chronic coronary artery disease with reversible perfusion defect detectable by single-photon emission computed tomography and a left ventricular ejection fraction (LVEF) less than 45%. Patients also had to be ineligible for revascularization, as assessed by coronary angiography. This study complied with the Declaration of Helsinki, local ethics committee approved the research, and all patients signed a written informed consent.
In this analysis, we compared outcomes between diabetics and nondiabetic patients. Diabetes mellitus was defined according to the World Health Organization Report,10 and patients either on diet or with pharmacologic treatment were included.
Bone Marrow Aspiration and Transendocardial Delivery of BMMNCs
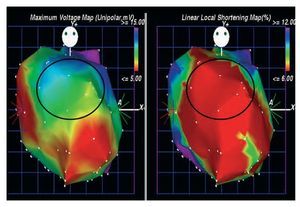
Bone marrow aspiration was performed following the standard technique.5 Ficoll-density centrifugation was used to isolate the mononuclear fraction of bone marrow.5 Before cell injection, electromechanical mapping was performed to target the specific treatment area (viable myocardium: unipolar voltage 36.9 mV) (Figure 1).5 Subsequently, 15 injections of 0.2 cc were carried out with the NOGA MYOSTAR injection catheter (Cordis Corporation, Miami Lakes, Fl, USA).
Figure 1. Electromechanical maps viewed from anterior-posterior position used to target the specific treatment area. The Unipolar voltage map (left) and the local shortening map (right). The marked zone (anterior wall) identified viable myocardium (unipolar voltage 36.9 mV), associated with decreased mechanical activity.
Clinical Follow-up and Non-Invasive Evaluation
Clinical evaluation and functional tests were performed using identical protocols5 at the time of the injection procedure and at 6 months' follow-up. Transthoracic echocardiography was used to assess LVEF and volumes and was interpreted at an independent, blinded core lab. Ramp treadmill protocol was performed using the standard incremental protocol of 0.5 mph initially, an inclination of 0% to 10%, and a planned exercise duration of 10 minutes.
Statistical Analysis
Statistical analyses were performed by using SPSS version 12.0 (SPSS Inc, Chicago, Ill). Quantitative data were presented as median (interquartile range) and were compared by non parametric test. Categorical variables were presented as percentages and were compared by means of Fisher exact test. A 2-tailedvalue of P<.05 was considered significant.
RESULTS
Baseline Characteristics
At 6-months, 25 of the 26 patients had completed the follow-up period; 1 refused to continue in the trial and was excluded from the analyses. Baseline characteristics were comparable between groups (Table 1), except for a trend towards younger and more previous myocardial infarctions in nondiabetic group. The area and location of injections were not significantly different between groups (Table 1).
Six-Month Follow-up
A median of 29.7 (28.2-30.0)´106 cells were injected. CD45lo CD34+ cells comprised 2.2% (1.6%-2.8%); viability was 96.9% (3.8%). BMMNCs characteristics were similar between groups (Table 1), except there was a trend toward a significant increase in natural killer cells in nondiabetic patients and higher viability rate of the injected cells in diabetic patients.
The echocardiographic data did not show any significant differences between groups in LVEF or enddiastolic volumes (Table 2). However, a significant decrease in end-systolic volume from baseline to follow-up was observed in nondiabetic patients. At 6-month follow-up, a significant improvement in both NYHA class (baseline vs 6-month, 3.0 [1.75-3.0] vs 1 [1.0-2.0], P=.04) and CCSAS (3.0 [2.0-4.0] vs 1.0 [1.0-1.5], P=.04) was observed in nondiabetic patients. Conversely, diabetic patients did not show any significant differences at follow-up: NYHA class, 3.0 (1.0-3.0) versus 2.0 (2.0-2.0), P=1; CCSAS, 3.0 (3.0-3.75) versus 2.0 (1.75-3.25), (P=.98).
Overall (diabetics + nondiabetics), there was a significant increase in VO 2max levels from baseline to follow-up (15.2 [10.9-18.4] vs 19.3 [14.7-25.2]; P =.01) and in metabolic equivalents (METs) (4.3 [3.1-5.3] vs 5.3 [3.9-7.4], P =.006). However, when diabetics and nondiabetic patients were separated, this significant increase in the VO 2max and MET levels was observed only in the nondiabetic (Table 2). No significant changes were seen in the diabetics group.DISCUSSION
This is the first preliminary evidence from a clinical stem cell study to show that transendocardial treatment with autologous BMMNCs possibly results in less functional and clinical improvement in diabetics than in nondiabetic patients with chronic ischemic cardiomyopathy. Several mechanisms may be involved to explain these findings. First, EPC function is impaired in diabetics. Tepper et al8 showed that in culture circulating EPCs proliferate less in diabetics than in nondiabetic patients. Secondly, monocytes, which play an important role in angiogenesis and arteriogenesis, are also altered in diabetic. Waltenberger et al9 demonstrated that monocytes from diabetic patients have an attenuated response to VEGF in a cell-migration assay. In this regard, there is also evidence of an increase in myocardial expression of VEGF, a decreased expression of its receptor, and a down regulation of its signal transduction in diabetics.11
Other mechanisms that have been suggested to explain the relative lack of angiogenesis in diabetics are the presence of advanced glycation end-products12 and glycation of circulating growth factors, reducing their biological function.13
Although previous preclinical and clinical studies have shown that BMMNCs may contribute to increased angiogenesis and perfusion in patients with chronic ischemia,5-7 the results from this analysis may suggest that diabetics patients have a less pronounced response to and a blunted benefit from cell therapy. Prospective, double-blind, placebo-controlled trials are warranted to further clarify this very important issue.
Limitations
There are several limitations of the present study. This is an observational study that came from 2 phase-I trials with small number of patients. As a result, it is not powered to detect efficacy and may be responsible for some baseline differences between the groups. The results derived should, therefore, be interpreted with caution. However, until now, no peer-reviewed studies have been published concerning the effects of stem cell therapy in this subset of high-risk patients.
Correspondence:
Dr E. Perin
Stem Cell Center at the Texas Heart Institute
St. Luke's Episcopal Hospital, Houston, TX, USA
E-mail: eperin@heart.thi.tmc.edu
Received May 31, 2007.
Acdepted for publication November 2, 2007.