Type 2 diabetes is a metabolic disorder associated with an increased risk of cardiovascular disease. According to estimates, approximately 700 million people worldwide will be living with diabetes by 2045.1 The growing incidence of this disease is linked to population aging and a disproportionate increase in obesity, in turn linked to an exponential increase in cardiovascular disease.
Type 2 diabetes has complex pathophysiological mechanisms and is associated with a number of metabolic disorders, including insulin resistance, chronic systemic inflammation, and elevated oxidative stress. These disorders are all risk factors for heart failure (HF) and cardiovascular disease. Recent studies of new antidiabetic drugs with significant cardiovascular benefits are revolutionizing the treatment of type 2 diabetes, prompting a shift from a largely glucocentric focus to a broader strategy spanning the cardiometabolic spectrum.
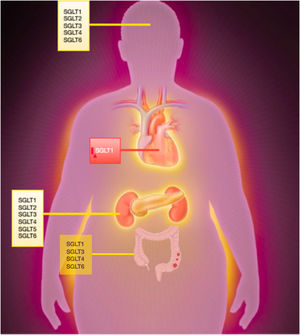
Sodium-glucose cotransporter receptorsSodium-glucose cotransporter (SGLT) receptors belong to the solute carrier 5 (SLC5) family, which comprises 12 distinct coreceptors. All SGLT receptors except SGLT3 are involved in the active transport of sodium and glucose.
SGLT 1-6 are the main glucose transporters, and within this group, SGLT1 and SGLT2 are the most widely studied receptors. Under normal physiological conditions, SGLT2 receptors are responsible for approximately 90% of glucose reabsorption in the kidneys (segments S1 and S2 of the proximal tubule). SGLT1 receptors (S3 segment) mediate the remaining 10%, as well as glucose reabsorption in the intestines. In certain circumstances, such as uncontrolled diabetes or SGLT2 inhibition, SGLT1 receptors play a greater role in renal glucose absorption.
SGLT1 is more widely distributed than SGLT2 and is present in the central nervous system and the myocardium, especially in ischemic conditions.
DiFranco et al.2 found SGLT1 to be overexpressed in human hypertrophic and ischemic hearts. Increased expression of this receptor has also been reported in patients with dilated cardiomyopathy, metabolic syndrome, and left ventricular assist devices (postimplantation). Although SGLT1 is essential for life, genetic variants resulting in reduced expression of this receptor appear to be associated with certain health benefits. Population-based studies, for instance, have shown links between diminished SGLT1 expression and resistance to ischemia-reperfusion injury and protective effects in the settings of diabetes and HF. Selective SGLT1 inhibitors, which partly inhibit SGLT1, may offer similar protection.
The other receptors in the SLC5 family regulate the transport of diverse substances, including myo-inositol (SLC5A3 and SLC5A11), iodine (SLC5A5), vitamins (SLC5A6), amino acids (SLC5A7), and monocarboxylate (SLC5A8 and SLC5A12) (figure 1).
SGLT2 inhibitorsAlthough SGLT2 inhibitors were initially developed as hypoglycemic agents, they have since become the fourth pillar in the management of HF. Their cardiovascular benefits, confirmed in a recent meta-analysis,3 include a significant reduction in cardiovascular events in both type 2 diabetes and across the spectrum of HF, irrespective of diabetes status.
The independent association between the cardioprotective and glucose-lowering effects of SGLT2 inhibitors was first observed in the EMPA-TROPISM trial (NCT03485222), conducted by our group to explore the mechanistic effects of empagliflozin in a nondiabetic population of patients with HF and reduced ejection fraction.4 After just 6 months of treatment with empagliflozin, the patients showed significant improvements in adverse ventricular remodeling (reduced ventricular volumes, mass, and sphericity, leading to improvements in ejection fraction).
Further research by our group suggests that SGLT2 inhibitors might also induce a transcriptional paradigm characterized by nutrient deprivation and hypoxia, increased ketosis, and improved iron metabolism.5 Selective use of ketone bodies as a source of energy improves myocardial energy efficiency. SGLT2 inhibitors also reduce epicardial adipose tissue in patients with HF and alter the signaling pathways of adipokines involved in inflammation and oxidative processes. Finally, both experimental and clinical models have shown that SGLT2 inhibitors decrease myofilament stiffness and extracellular matrix remodeling/fibrosis in the heart, thereby improving diastolic function.6
Dual SGLT1-2 inhibitorsSotagliflozin is the most selective, but not the only, inhibitor with an effect on SGLT1. It has a 20× selectivity for SGLT2 over SGLT1, compared with a 2500× selectivity for empagliflozin, a 1200× selectivity for dapagliflozin, and a 250× selectivity for canagliflozin.7 Canagliflozin, the SGLT2 inhibitor with the greatest impact on SGLT1, was recently shown to have cardioprotective properties. It was mainly found to reduce oxidative stress, an effect attributed to its action on SGLT1.8 Similar effects were observed in the CANVAS Program, which showed a marked, though nonsignificant, trend towards a reduction in atherothrombotic events in patients treated with canagliflozin.
Inhibition of intestinal SGLT1 may directly reduce postprandial hyperglycemia, a recognized risk factor for HF. Improved postprandial blood glucose and glycohemoglobin values, for example, have been observed in rats treated with a selective SGLT1 inhibitor.9 Intestinal SGLT1 inhibition would result in more glucose reaching the colon, stimulating the secretion of glucagon-like peptide 1 (GLP-1) by the microbiome. The probable mechanism involved would be the breakdown of glucose into short-chain fatty acids, which have been linked to the synthesis and indirect expression of GLP-1.
The cardiometabolic benefits observed for sotagliflozin in the SOLOIST-WHF (NCT03521934)10 and SCORED (NCT03315143)11 trials have sparked interest in the use of dual SGLT1-2 inhibitors, as it is thought that a dual approach might be more beneficial than SGLT2 inhibition alone.
In the SOLOIST-WHF trial comparing sotagliflozin and placebo in patients with type 2 diabetes who had recently experienced decompensated HF, sotagliflozin initiated before or shortly after discharge was associated with a 33% reduction in the composite endpoint of deaths from cardiovascular causes and hospitalizations and emergency room visits for HF and a 36% reduction in hospitalizations for HF. No significant reductions were observed for cardiovascular or all-cause deaths. In the SCORED trial comparing sotagliflozin and placebo in patients with type 2 diabetes and chronic kidney disease with or without albuminuria, sotagliflozin was associated with a 26% reduction in deaths from cardiovascular causes and hospitalizations and emergency room visits for HF. Notably, sotagliflozin is the first dual SGLT1-2 inhibitor to be linked to a reduction in atherothrombotic events (> 30% reduction in the incidence of fatal and nonfatal stroke and acute myocardial infarction [AMI]).
The US Food and Drug Administration recently approved sotagliflozin, together with a number of SGLT2 inhibitors, for the treatment of HF. Although the SOLOIST-WHF10 and SCORED11 trials did not include patients with diabetes and, in addition, had short median follow-up times, their findings supported this controversial approval, granted without consideration of ejection fraction, chronic kidney disease, or even glycemic status. The foundation for FDA approval was the anticipated class effect of SGLT2 inhibitors, associated with the significant cardioprotective and promising atherothrombotic effects described to date (table 1). This class effect will probably also form the basis of future approvals (eg, by the European Medicines Agency) and lay the groundwork for promising research in the field of HF.
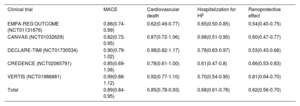
Class effect of sodium-glucose cotransporter 2 inhibitors
| Clinical trial | MACE | Cardiovascular death | Hospitalization for HF | Renoprotective effect |
|---|---|---|---|---|
| EMPA-REG OUTCOME (NCT01131676) | 0.86(0.74-0.99) | 0.62(0.49-0.77) | 0.65(0.50-0.85) | 0.54(0.40-0.75) |
| CANVAS (NCT01032629) | 0.82(0.72-0.95) | 0.87(0.72-1.06) | 0.68(0.51-0.90) | 0.60(0.47-0.77) |
| DECLARE-TIMI (NCT01730534) | 0.90(0.79-1.02) | 0.98(0.82-1.17) | 0.78(0.63-0.97) | 0.53(0.43-0.66) |
| CREDENCE (NCT02065791) | 0.85(0.69-1.06) | 0.78(0.61-1.00) | 0.61(0.47-0.8) | 0.66(0.53-0.83) |
| VERTIS (NCT01986881) | 0.99(0.88-1.12) | 0.92(0.77-1.10) | 0.70(0.54-0.90) | 0.81(0.64-0.70) |
| Total | 0.89(0.84-0.95) | 0.85(0.78-0.93) | 0.68(0.61-0.76) | 0.62(0.56-0.70) |
HF, heart failure; MACE, major cardiovascular adverse events; NCT, National Clinical Trial.
Stroke and AMI prevention is crucial in patients with type 2 diabetes, but SGLT2 inhibition has not yet been linked to a reduction in either stroke or AMI. Epidemiological studies have traditionally linked reductions in inflammatory biomarkers, oxidative stress, visceral obesity, and systolic blood pressure to a reduced incidence of stroke. This benefit, however, has not been observed in patients treated with SGLT2 inhibitors, perhaps, in part, because these drugs stimulate erythropoietin production, resulting in increased hematocrit and blood viscosity.
Recent meta-analysis data have shown a modest reduction (≈ 10%) in AMI incidence in patients treated with SGLT2 inhibitors.12 According to porcine studies, empagliflozin is capable of reducing infarct size, largely via a lowering of apoptosis and oxidative stress.4 It should be noted, however, that the improvements observed are largely linked to decreased myocardial oxygen demands (ie, there are no direct effects on atherosclerosis).
Promising results have been described for dual SGLT1-2 inhibitors in the context of stroke and AMI. As mentioned, the SCORED trial showed a significant association between sotagliflozin and a reduced risk of both stroke and AMI. Understanding of the underlying mechanisms is limited, however, as multiple factors have been linked to AMI and stroke development in patients with diabetes (eg, insulin resistance, vascular calcification, endothelial dysfunction, and inflammation).
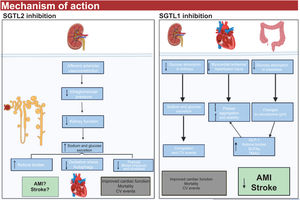
A number of mechanisms of action have been proposed to explain the cardiovascular benefits of dual SGLT1-2 inhibitors (figure 2).
- •
Dual SGLT1-2 inhibition is associated with increased endogenous GLP-1 levels, which, in preclinical studies, have been linked to plaque stabilization and a slowing of thrombus growth.13
- •
Disruption of the microbiome as a result of SGLT1 inhibition is crucial. Patients with obesity, type 2 diabetes, inflammation, and HF experience a reduction in short-chain fatty acid levels. This reduction subsequently leads to an increase in bacteria and lipopolysaccharides, which are strongly associated with coagulation disorders.12
- •
The microbiome also produces trimethylamine n-oxide (TMAO), a metabolite linked to increased cardiovascular risk. A reduction in TMAO production might explain some of the beneficial effects observed for SGLT1-2 inhibitors.13
- •
SGLT1-2 inhibition causes an overproduction of ketone bodies, potentially contributing to the cardioprotective effects of dual inhibitors, as an increase in ketone bodies could directly improve myocardial energy efficiency.5
Selective sodium-glucose cotransporter inhibitors types 1 (SGLT1) and 2 (SGLT2) and possible mechanisms of action. AMI, acute myocardial infarction; BP, blood pressure; CV, cardiovascular; GLP-1, glucagon-like peptide 1; ROS, reactive oxygen species (oxidative stress); SCFA, short-chain fatty acids; TMAO, trimethylamine N-oxide.
One of the main limitations of dual SGLT1-2 inhibitors is the relatively limited experience with their use. Mean exposure to the drugs in the 2 main trials conducted to date is just 14.112 patient-years10,11 compared with 39.798 patient-years for SGLT2 inhibitors. Treatment duration in HF is also considerably shorter for sotagliflozin (917 patient-years) than for either dapagliflozin (21 602 patient-years) or empagliflozin (18 214 patient-years).
The adverse effects reported for dual SGLT1-2 inhibitors are similar to those described for SGLT2 inhibitors. Genital fungal infections are the most common effect described to date. Hypoglycemia and diabetic ketoacidosis are rare. Adverse effects, however, have only been described in patients with diabetes to date. Tolerability of SGLT1-2 inhibitors in other patient populations has yet to be determined.
Other SGLT receptorsAlthough relatively little is known about the other receptors in the SGLT family, there are a number of key aspects to highlight14:
SGLT3. This receptor is predominantly found within the autonomic nervous system in the digestive tract, where it serves as a glucose sensor. Its expression is reduced in obesity, and it may have a role in metabolic syndrome. Certain SGLT3 inhibitors (dapagliflozin, ertugliflozin, and sergliflozin etabonate) can affect SGLT3 expression in the brain. The clinical significance of this inhibition, however, is as of yet unknown.
SGLT4. This receptor primarily functions as a mannose transporter and is mainly expressed in the small intestine, kidney, brain, and liver. SGLT4 has been ascribed a potentially pathogenic role in proliferative diabetic retinopathy. Little is known about its therapeutic potential.
SGLT5. This receptor is expressed in the proximal tubule, and like SGLT3, it contributes to fructose absorption. It has been linked to lipid metabolism disorders
SGLT6. This receptor is a myo-inositol transporter expressed mainly in the kidneys. It has been linked to autoimmune and psychiatric disorders.
Future perspectivesEmerging therapeutic agents such as SGLT2 inhibitors, dual SGLT1-2 inhibitors, and GLP-1 receptor agonists have demonstrated their ability to achieve metabolic targets beyond glycemic control. The biological effects of these drugs appear to stem from complementary mechanisms of action. Inhibition of SGLT1, which is widely distributed through the body, may amplify the benefits of SGLT2 inhibitors, notably through the stimulation of GLP-1 production. Comparative studies of these drugs are paving the way for new and promising directions in clinical research. Also of interest is research into the simultaneous or sequential use of selective or dual SGLT inhibitors and GLP-1 receptor agonists, particularly in patients at high cardiovascular risk.
Finally, the presence of SGLT receptors in the brain and their potential effects on stroke offer further avenues for research. Both SGLT1 and SGLT2 are expressed in the central nervous system, where they facilitate the transport of glucose, galactose, and sodium. Experimental evidence (to date from murine studies only) suggests that SGLT1 inhibition following a brain injury may reduce lesion size and edema, and possibly even lead to cognitive improvements.15 This potential neuroprotective effect also heralds new investigative challenges in the field.
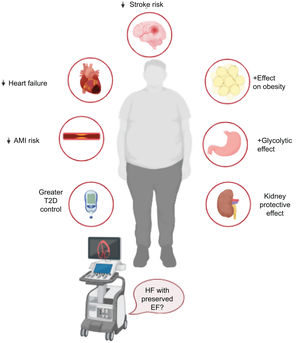
Clinical applicationsWhile the mechanisms of action underlying SGLT2 and SGLT1-2 inhibition in the field of HF remain to be determined, the effects of these drugs on ventricular remodeling, epicardial fat, vascular stiffness, fibrosis, and inflammation, all known determinants of diastolic dysfunction, could position SGLT2 and dual SGLT1-2 inhibitors as potential treatments for patients with HF and preserved ejection fraction. Dual inhibitors, with their reported atherothrombotic effects (stroke and AMI), greater glycolytic capacity, and effects on kidney function (SCORED trial), are also positioned, perhaps even ahead of SGLT2 inhibitors, as promising primary and secondary preventive treatments for patients at high and very high cardiovascular risk, particularly those with type 2 diabetes, obesity, and chronic kidney disease. The SOTA-P-CARDIA trial (NCT05562063) will explore the efficacy and mechanism of action of sotagliflozin in nondiabetic patients with HF and preserved ejection fraction and hopefully answer some key questions (figure 3).
In summary, sotagliflozin is the first dual SGLT1-2 inhibitor to show clear cardiovascular benefits. The promising results to date have sparked interest in the dual inhibition approach and the potential ability of dual inhibitors to contribute to (or even enhance) the cardiovascular benefits of SGLT2 inhibitors. The additional potential of SGLT1-2 inhibitors to reduce stroke and AMI in patients with high cardiovascular risk positions them as key treatments in this population and patients with existing cardiovascular disease.
FUNDINGNone
CONFLICTS OF INTERESTNo conflicts of interest.