Modulation of vascular tone is one of the most relevant estrogen effects. A beneficial effect on endothelial function in postmenopausal women has also been proposed for the selective estrogen receptor modulator raloxifene. However, its effects in women with established cardiovascular disease have not been fully elucidated. In addition, recent trials have generated controversy regarding thromboembolic risk with raloxifene use. The aim of the study was to assess the effect of raloxifene on: a) endothelial function and b) coagulation and fibrinolysis pathways.
MethodsThe MERCED trial was a prospective, randomized clinical trial. Thirty-three postmenopausal women with ischemic heart disease were enrolled in the study. Raloxifene treatment was administered for a 3-month period, according to a double-blind crossover design. Assessment of vascular function and biologic parameters related to coagulation pathways were conducted at various pre-established time-points.
ResultsFlow-mediated dilatation was severely impaired in the study population, and raloxifene had no effect on endothelial function. Treatment with raloxifene was associated to decreased levels of fibrinogen (3.41 [3.11-3.74] vs. 3.69 [3.40-4.00], P<.05); prothrombin fragments F1+2 (0.93 [0.77-1.12] vs. 0.94 [0.78-1.15], P<.05); and plasmin/antiplasmin complexes (211 [166-267] vs. 242 [199-295], P<.01).
ConclusionsThe present study provides evidence that in postmenopausal women with demonstrated endothelial dysfunction and ischemic heart disease, mid-term treatment with raloxifene does not affect endothelial function. In the MERCED trial, no increased thrombotic risk was observed, but a decreased thrombotic and fibrinolytic activity was observed with raloxifene. Further studies are required to determine whether thrombotic risk is associated with specific clinical characteristics or subgroups of postmenopausal women with cardiovascular disease.
Keywords
.
INTRODUCTIONModulation of vascular tone is one of the most relevant effects of estrogens.1 Intracoronary or percutaneous estrogen administration has been shown to restore the vasodilator response to acetylcholine, both in patients with atherosclerotic coronary artery disease and those with angiographically normal coronary arteries but with proven endothelial dysfunction.2, 3, 4
Since the publication of the HERS study,5 demonstrating an increased risk in thromboembolic complications and breast cancer in postmenopausal women treated with a combination of estrogen plus progesterone, there has been growing interest in evaluating the effects of the selective estrogen receptor modulators in the cardiovascular system.
A beneficial effect on endothelial function has also been proposed for the selective estrogen receptor modulator (SERM) raloxifene.6, 7 More specifically, a previous clinical study in postmenopausal women receiving raloxifene for 12 months demonstrated an increased brachial artery endothelial function as compared with control patients. From a thrombosis point-of-view, raloxifene has been reported to modify coagulation parameters. On one hand, a favorable decrease in fibrinogen levels has been reported in postmenopausal women8; on the hand, an increase in procoagulant parameters has been documented with raloxifene mid-term treatment.9 No previous studies analyzing coagulation and fibrinolysis pathways in women with established cardiovascular disease have been conducted.
The MORE study10 had shown a decreased risk of cardiovascular events in a subgroup of postmenopausal women at increased cardiovascular risk. The RUTH study sought to analyze the effect of raloxifene on women with demonstrated ischemic heart disease or at increased risk, but failed to demonstrate any reduction of the risk of coronary heart disease. In addition, an excess of fatal stroke and thromboembolic events was observed. No assessment of coagulation and fibrinolysis parameters was done in the RUTH study.11
We undertook the MERCED trial with the aim to study the effects of raloxifene on endothelial function in postmenopausal women with coronary heart disease. In addition, to assess thrombosis risk, a thorough analysis of coagulation and fibrinolysis pathways has been conducted.
METHODS Study DesignThe MERCED study was a 2-center, national, randomized, double-blind, cross-over trial. Treatment sequence allocation (raloxifene-placebo; placebo-raloxifene) was randomly assigned by blocks of 4 patients for both participating centers. Patients, their treating physicians, and the investigators performing endothelial function analysis in each center were blinded to treatment sequence allocation. Study design follows the CONSORT statement for reporting randomized trials.
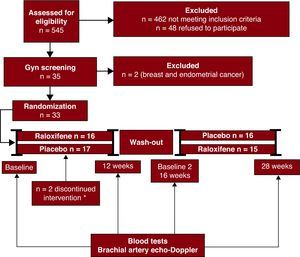
Study duration was 28 weeks, divided in 3 phases: a 12-week period during which patients were randomly assigned with the use of a computer-generated table to receive either raloxifene 60mg/day or placebo, followed by a 4-week wash-out period, and a final 12-week period in which the patients switched to the corresponding placebo or raloxifene. A balanced permuted-block approach (in blocks of 4 patients) was used to prepare the randomization tables for each participating center. Randomization tables were provided by the Unidad de Ensayos Clínicos of the Hospital Clínic, and study medications were provided by the pharmacy department at our institution. Figure 1 illustrates the study flowchart.
Figure 1. Study flowchart. Enrollment strategy for the MERCED trial. Eligible patients had evidence of ischemic heart disease and were required to have a normal gynecological screening. Randomized patients were allocated to receive placebo or raloxifene, in a double-blinded manner during the first phase of the study, and switched to the other treatment arm for the second phase, after a 4-week washout period. *2 patients randomized to placebo discontinued treatment due to medication intolerance.
The protocol was approved by the local ethics committee of each participating center and the Agencia Española del Medicamento..
Analysis of blood samples for biochemistry and coagulation was conducted in a centralized manner. Endothelial function analysis was performed at each participating center using the same study protocol and sonographic equipment and software. Measurements of endothelial function parameters were also centrally evaluated.
Study PopulationConsecutive postmenopausal women (plasma estradiol levels<30 pg/mL and FSH>40 UI/L), aged ≤70 years, admitted to the cardiology department of the participating centers, with documented coronary artery disease (at least 1 vessel with a stenosis >70% or history of a previous myocardial infarction) were assessed for eligibility. Exclusion criteria were contraindications to raloxifene (previous venous thrombotic disorders, hepatic disease, breast or endometrial cancer), chronic renal failure (creatinine >2mg/dL), participation in a clinical trial<30 days prior to randomization or having received hormone replacement therapy in the previous 6 months. All patients gave written informed consent. Before enrollment, patients underwent a thorough gynecological evaluation consisting of a pelvic exam, pap smear and mammography in all patients, and pelvic sonography when indicated.
Endothelial Function AssessmentEndothelial function was studied using high-resolution ultrasound of the brachial artery, according to a previously validated technique.3, 12 Blind analysis of all images was conducted centrally by a blinded investigator.
The studies were carried out in a quiet, temperature-controlled room (24°C) with the patient in the supine position, using a high resolution vascular probe connected to a conventional ultrasound. All studies were performed at the same time of day, and patients rested for at least 10min prior to beginning the study.
A longitudinal section of a nontortuous segment of the right brachial artery 2cm to 5cm above the elbow was scanned. The center of the artery was identified by obtaining the clearest image of the anterior and posterior arterial wall layers. Each scan consisted of a longitudinal image of the brachial artery and a pulsed wave Doppler spectral display of the brachial artery flow. Endothelial-dependent vasodilation was assessed by analysis of the brachial artery diameter changes in response to an increase in flow. Reactive hyperemia was achieved by the rapid release of a pneumatic pressure cuff placed around the forearm, distal to the arterial segment scanned, which was inflated up to 300mmHg during 4 to 5min. A pulsed wave Doppler signal of the brachial artery flow and bidimensional images were recorded 55 s to 65 s after cuff release.
Blind analysis of all images was conducted centrally by a blinded investigator. Staff at each participating center was specifically trained to perform the endothelial function analysis in a standardized protocol.
Flow-mediated vasodilation (FMD) was used as an index of endothelium-dependent vasodilation and was calculated as the percentage change in brachial artery mean diameter after reactive hyperemia over that obtained at baseline. Flow was estimated from the velocity-time integral of the pulsed wave Doppler signal and heart rate. Vasodilation induced by sublingual nitroglycerine was used as an index of endothelium-independent vasodilation.
Using this methodology and a nested analysis of variance, inter-observer and intra-observer variance for brachial artery diameter measurement in our laboratory has been reported to be 0.0002 (0.04% of total variability) and 0.001 (0.22% of total variability), respectively.
Blood TestsRaloxifene effects on the following variables were analyzed: lipid profile (total cholesterol, high density lipoprotein cholesterol [HDLc] and low density lipoprotein cholesterol [LDLc], apo A, apo B, lipoprotein [a] and triglycerides); coagulation (F 1+2, activated factor VII and factor XII, and thrombin generation curves); and fibrinolysis (plasminogen activator inhibitor type I [PAI-1], thrombin-activatable fibrinolysis inhibitor [TAFI] antigenic and activity, plasmin-antiplasmin [PAP] complexes, and clot lysis assay). Blood samples were obtained at baseline (time 0), and at weeks 12, 16, and 28.
AssaysGeneral laboratory work, including hematology and lipid profile, was performed following standard methodology.
Coagulation ParametersProthrombin and activated partial thromboplastin times were determined in an automated coagulometer CA-1500 (Dade Behring, Marburg, Germany) using standard reagents (Thromboplastin IS and Actin FSL; Dade Behring) and were expressed as ratios (patient time:control time). Fibrinogen level was measured by the Clauss’ technique. The prothrombin fragments F1+2 were assessed as a thrombin generation marker by ELISA (Enzygnost-F1+2; Dade Behring). Activated factor XII was determined by a direct immunoassay (Shield Diagnostics, Dundee, United Kingdom), and activated factor VII was determined by ELISA (American Diagnostica, Greenwich, Connecticut, United States). Thrombin generation curves were measured as endogenous thrombin potential (ETP) using a chromogenic substrate method (Dade-Behring) in a fully automated coagulometer (BCS-XP, Dade-Behring).
Fibrinolysis ParametersPAI-1 plasma antigen was measured by ELISA, based on a double antibody principle (Imulyse PAI-1, Biopool, Umea, Sweden). TAFI plasma antigen was measured by ELISA (Asserachrom TAFI, Stago), and the plasma activity related to TAFI was quantified by a chromogenic method (STA-Stachrom TAFI, Stago). Plasma levels of plasmin–alpha 2-antiplasmin complexes were quantified as a plasmin generation marker by ELISA (Dade Behring).
Clot lysis time was studied in a plasma system in which tissue plasminogen activator-mediated fibrinolysis of a thrombin-induced clot is measured using changes in turbidity at 405nm (Multiskan Ascent, Thermo Labsystems, Finland). The clot lysis time is expressed as percentage of the normal control.
Samples from the study population were compared with healthy controls, obtained from a representative population sample (n=99 women, aged 59±6 years), from which we already have anonymized serum samples in our hospital's laboratory database.
Sample Size and Statistical AnalysisAssuming a standard deviation of 2.76% and to detect a 2% difference in the dilatation of the humeral artery, 30 patients were needed to achieve a statistical power 1-β=80%, α=0.05 in a 2-sided test.
We assessed the normality of variables by examining the normal probability plots. The systolic blood pressure at baseline, reactive hyperemia, prothrombin time, activated partial thromboplastin time, fibrinogen, PAP complexes, F1+2, HDLc, triglycerides and glucose were log-transformed to achieve normality.
We used the Student t-test or Mann-Whitney U-test, as appropriate, to determine differences in baseline characteristics. We checked the possible carryover effect by testing a period-by-treatment interaction term in the general linear mixed models. Because the period-by-treatment interaction term was not statistically significant in any model, we did not include period-by-treatment terms in the final models used. These models used the differences between pre- and posttreatment values as the dependent variables and included independent variables of treatment as fixed effects, and participant number as random effect.13 In tables, the log-transformed variables were reversed to get original values; therefore, differences become ratios and means are geometric instead of arithmetic. Statistical significance was defined as a P value<.050 for a 2-sided test. We performed analyses by using the R software (R Development Core Team 2006; R=A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria).
RESULTSIn total, 33 postmenopausal women with demonstrated ischemic heart disease were enrolled in the study. During the study follow-up, 2 patients —randomized to placebo— withdrew consent due to gastrointestinal intolerance to study medication. Overall, raloxifene was well tolerated and no significant side effects were observed.
Baseline characteristics of the study population are summarized in Table 1.
Table 1. Baseline Characteristics of the Study Population.
| Age (years) | 59.7±5.91 |
| Hypertension | 71.0 |
| Diabetes mellitus | 22.6 |
| Dyslipidemia | 74.2 |
| Smoker or exsmoker ≤1y | 19.4 |
| Family history of IHD | 53.3 |
| Medical treatment at study entry | |
| Nitrates | 38.7 |
| ASA | 73.3 |
| ACEI | 38.7 |
| Statins | 87.1 |
| Betablockers | 67.7 |
| Clopidogrel | 32.3 |
| Oral anticoagulants | 3.2 |
| Calcium channel blockers | 38.7 |
| ARA II | 3.2 |
| SBP(mmHg) | 136±22.5 |
| DBP(mmHg) | 76.5±10.7 |
| Type of coronary event | |
| STEMI | 45.2 |
| Angina and nonSTEMI | 54.8 |
| Previous history of CVD | |
| AMI | 38.7 |
| PCI | 61.3 |
| CABG | 6.45 |
| Angina | 58.6 |
| CVA | 3.2 |
| Peripheral arterial disease | 10.3 |
ACEI: angiotensin-converting enzyme inhibitors; AMI: acute myocardial infarction; ARA: angiotensin receptor antagonists; ASA: acetylsalicylic acid; CABG: coronary artery by-pass grafting; CVA: cerebrovascular accident; CVD: cardiovascular disease; DBP: diastolic blood pressure; IHD: ischemic heart disease; PCI, percutaneous coronary intervention; SBP: systolic blood pressure; STEMI: ST elevation myocardial infarction.
Data are expressed as percentage or as mean±standard deviation.
During the study follow-up, no differences were observed regarding the control of blood pressure, dyslipidemia or diabetes with raloxifene or placebo. Likewise, no significant differences in acute ischemic events, angina functional class, or other clinical events were observed between treatment groups.
All data regarding clinical and biologic parameters analyzed are summarized in Table 2.
Table 2. Effect of Raloxifene Treatment on Lipid Profile, Endothelial Function, Thrombosis and Fibrinolysis.
| Pre-Raloxifene | Post- Raloxifene | P | |
| Lipids / Glucose | |||
| Total cholesterol (mg/dL) | 174 (160-188) | 177 (162-191)] | NS |
| HDLc (mg/dL) | 46.5 (40.8-53.1) | 47.0 (40.7-54.4) | NS |
| LDLc (mg/dL) | 102 (91.1-113) | 104 (90.7-117) | NS |
| Triglycerides (mg/dL) | 104 (86.3-125) | 104 (86.6-125) | NS |
| Glucose (mg/dL) | 104 (92.3-118) | 102 (91.0-114) | NS |
| Endothelial function | |||
| Baseline BA diameter (mm) | 3.58 (3.41-3.75) | 3.57 (3.40-3.74) | NS |
| Post-hyperhemia BA diameter (mm) | 3.63 (3.48-3.79) | 3.65 (3.49-3.80) | NS |
| Dilatation post-hyperemia (%) | 2.16 (1.27-3.05) | 2.83 (1.82-3.83) | NS |
| Post-NTG BA diameter (mm) | 4.30 (4.17-4.42) | 4.29 (4.16-4.43) | NS |
| Reactive hyperemia (mL/min) | 363 (314-420) | 317 (268-375) | NS |
| Thrombosis | |||
| Fibrinogen (g/L) | 3.69 (3.40-4.00) | 3.41 (3.11-3.74) | <.05 |
| F 1+2 (nmol/L) | 0.94 (0.78-1.15) | 0.93 (0.77-1.12) | <.05 |
| Factor Xlla (ng/mL) | 3.33 (2.90-3.77) | 3.03 (2.62-3.44) | NS |
| Factor Vlla (ng/mL) | 5.09 (4.47-5.71) | 5.36 (4.67-6.06) | NS |
| Thrombin generation curve (Me) | 416 (391-441) | 402 (372-432) | NS |
| Fibrinolysis | |||
| PAI-1 (ng/mL) | 45.0 (36.2-53.8) | 50.6 (42.1-59.2) | NS |
| TAFI antigen (μg/mL) | 122 (112-132) | 118 (111-125) | NS |
| TAFI activity (%) | 122 (113-130) | 121 (112-130) | NS |
| Plasmin/antiplasmin complexes (μg/L) | 242 (199-295) | 211 (166-267) | <0.01 |
| Clot lysis assay (%) | 112 (102-122) | 114 (103-125) | NS |
BA: brachial artery; HDLc: high density lipoproteins cholesterol; LDLc: low density lipoproteins cholesterol; NTG: nitroglycerin; PAI: plasminogen activator inhibitor; TAFI: thrombin-activatable fibrinolysis inhibitor.
Data are displayed as the arithmetic mean (95% confidence interval) of the post- and pretreatment values.
Baseline median FMD in our study population was 1.81% (95% confidence interval: 1.13%±2.50%).
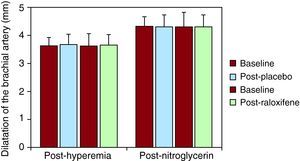
Among the different parameters analyzed assessing endothelial function, there were no differences between brachial artery diameter at baseline, or after raloxifene treatment. Blood flow during reactive hyperemia was unchanged before and after placebo or raloxifene. The percentage of increase in the brachial artery diameter during reactive hyperemia was higher after raloxifene treatment (2.83% vs. 2.16%), but no significant difference was demonstrated. All data are summarized in Table 2.
In addition, raloxifene treatment had no effect on endothelial-independent vasodilatation in response to nitroglycerin (Figure 2).
Figure 2. Vascular function. Assessment of brachial artery diameter post-hyperemia and after sublingual nitroglycerin. Flow-mediated dilatation of the brachial artery after hyperemia in baseline conditions, and after placebo and raloxifene treatment, as well as flow-mediated dilatation after nitroglycerin administration.
CoagulationLevels of F1+2, factor VIIa, factor XIIa, and fibrinogen were increased at baseline, when compared with healthy controls from our hospital's laboratory database (1.11±0.53 vs. 0.76±0.21 nmol/L; 5.09±1.8 vs. 2.8±0.8 ng/mL; 3.1±1.1 vs. 2.7±1.0 ng/mL; and 3.75±0.94 vs. 3.1±0.9g/L, respectively, all P<.05).
Raloxifene induced a decrease in fibrinogen levels compared with placebo (P<.05; Table 2).
Thrombin generation, explored through F1+2 levels, was decreased by raloxifene treatment compared with placebo (P<.05; Table 2).
FibrinolysisBaseline levels of PAI-1, TAFI antigen and TAFI activity were increased compared to healthy controls (50±23 vs. 22.8±12.4 ng/mL; 12.4±2.6 vs. 11.1±1.9μg/mL; and 123±23 vs. 111±10%, respectively, all P<.05).
A decrease in PAP complexes was observed in response to raloxifene treatment when compared with placebo (P<.01, Table 2), indicating reduced fibrinolytic activity.
DISCUSSIONThe MERCED trial did not demonstrate a beneficial effect of raloxifene on endothelial function in postmenopausal women with ischemic heart disease. In addition to endothelial function, as a secondary aim the present study thoroughly assessed the effects of raloxifene on thrombosis and fibrinolysis pathways, showing a decrease of several thrombotic risk markers.
Raloxifene and Endothelial FunctionEndothelial dysfunction is a risk factor for future cardiovascular events. In healthy women without obstructive coronary artery disease presence of endothelial dysfunction has been shown to predict ischemic heart disease.14 Improvement of endothelial function is one of the most studied mechanisms through which estrogens and SERMs exert the described beneficial effects on vascular tone modulation.
In our study, raloxifene treatment had no effect on endothelial-dependent or –independent vasodilatation.
Baseline median FMD in our study population was lower than that observed in previous studies of postmenopausal women with coronary heart disease,15 suggesting a more severe endothelial dysfunction in our population. A high proportion of our population had dyslipidemia and hypertension, together with a long-term history of coronary heart disease in most patients, a risk profile that very likely accounts for the severely FMD observed.
In regard to the effects of raloxifene on endothelial function, previous studies have reported divergent conclusions. In a study conducted in healthy postmenopausal women, with a baseline vasodilatory response of 8%, FMD endothelium-dependent FMD of the brachial artery increased after 6 months under raloxifene therapy, to the same extent as estrogens.16 In contrast to this previous study, our study population included women with coronary artery disease and a high risk factor burden. A likely explanation for the observed differences between both studies would be that raloxifene is not able to improve FMD in the presence of severe endothelial dysfunction. Similar to our work, a study conducted in postmenopausal women with known coronary artery disease and impaired FMD (2.84% at baseline), did not find any beneficial effect of raloxifene on endothelial function.15 Several findings of this previous study are consistent with ours: short duration of treatment (8 and 12 weeks, respectively), the risk factor burden of the study population, and demonstrated coronary artery disease. In the presence of advanced atherosclerosis with demonstrated coronary artery disease, the beneficial effects of estrogen receptor modulation via increase of NO production may be hampered, as eNOS levels are reduced in atherosclerosis17. Taken altogether, the results from previous and present studies firmly suggest that raloxifene is not able to improve endothelial function in postmenopausal women with established ischemic heart disease. This fact could also explain the failure of replacement therapy and raloxifene in this population in previous trials.5, 11
Linked to endothelial function, in the MERCED study no changes in the lipid profile were observed with raloxifene use. All patients were under statin treatment and mean levels of total cholesterol and LDLc were within recommended range, suggesting that in a population under adequate treatment with statins, raloxifene does not induce any further reduction in cholesterol levels. This finding is also consistent with the results from the previously discussed study conducted in women with coronary artery disease under optimal medical treatment.15
Raloxifene and ThrombosisThe clinical implications of the RUTH trial11 in terms of increased risk of death from stroke and venous thromboembolism do not encourage the use of raloxifene, even though it did not result in an increased incidence of coronary events. No assessment of thrombosis-related biologic parameters was performed in the RUTH trial, as we did in the present study. In our study, no adverse cardiovascular events were observed with raloxifene treatment, although the present study had a short follow-up time and small sample size.
Different disarrangements of the coagulation/fibrinolysis pathways have been previously described in patients with ischemic heart disease.18, 19, 20 Levels of PAI-1, F 1+2, factor VIIa, factor XIIa, fibrinogen, and levels of TAFI antigen and activity were higher in our study population when compared to a healthy population.
Treatment with raloxifene resulted in a decrease in fibrinogen levels. Raloxifene has been previously reported to decrease plasma levels of fibrinogen in healthy postmenopausal women.8 In our study, a substantial reduction in fibrinogen (0.92g/L on average) was observed with raloxifene. The pre-established follow-up of our study was 7 months; therefore, we cannot assess whether the observed decrease in fibrinogen levels would translate into a reduction of future cardiovascular events.
F1+2 levels, an indicator of thrombin generation, were increased at baseline in our population and decreased with raloxifene treatment. Previous studies conducted in healthy postmenopausal women have shown either no change or an increase in F1+2 levels in response to raloxifene.8, 9 The association of high fibrinogen levels with an increased thrombotic risk has been partially related to an altered generation of activated protein C.21 Increased levels of fibrinogen can impair protein C activation and, thus, thrombin generation. This mechanism may provide a link between the observed decrease in fibrinogen levels and thrombin generation, assessed through F1+2 levels, found in our study.
Raloxifene and FibrinolysisLevels of fibrinolysis regulators correlate with cardiovascular events in patients with risk factors.18, 19 More recently, the TAFI has been associated with the presence of cardiovascular risk factors, raising the possibility that increased TAFI activity or antigen levels may have a role in coronary artery disease.20, 22
The present study demonstrates the presence of a prothrombotic and hypofibrinolytic state, at baseline, in this population of postmenopausal women with ischemic heart disease.
Determination of PAP complexes in our study was aimed at further examining the fibrinolytic system in the context of ischemic heart disease. Increased levels of PAP have been associated with a higher risk of myocardial infarction and cardiovascular death.23 To our knowledge, this is the first study to assess the effect of raloxifene on PAP. Treatment with raloxifene resulted in a decrease in PAP complexes. We hypothesize that the decrease in PAP complexes found in our study may not reflect a decreased fibrinolytic activity per se, but is more likely a consequence of the decrease in thrombin generation, as it parallels the observed decrease in F1+2 levels.
Study LimitationsBecause of the small sample size and short duration of treatment, we were not able to detect changes in clinical variables during follow-up. It is possible that the observed effect of raloxifene is influenced by the concomitant medications the patients were receiving. However, given the randomized, cross-over design of the study, the possible confounder effect of medical treatment is randomly distributed among groups. A longer duration of treatment and extended follow-up would be needed in order to evaluate the long-term effects of the observed findings on thrombosis.
CONCLUSIONSOverall, the present study provides evidence that in postmenopausal women with demonstrated endothelial dysfunction and ischemic heart disease, mid-term treatment with raloxifene does not affect endothelial function. In addition, a decreased thrombotic activity was observed. The findings of the present study are controversial, given the previous evidence on increased thrombotic risk associated with raloxifene use. Whether thrombotic risk is increased in specific postmenopausal population subgroups remains to be assessed in future studies.
FUNDINGThis work was funded by the Red HERACLES grant (RD06/0009/0008), from the Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo (Spain).
CONFLICTS OF INTERESTNone declared.
Acknowledgements
We are greatly indebted to Dr. Magda Heras for participation in study design and critical review of the data and manuscript; to I. Ramió and T. Martorell for assistance in recruitment and follow-up of patients; to E. Martí for assistance in the statistical analysis; to E. Lilly for editorial assistance; and to L. González for secretarial assistance.
Received 5 October 2010
Accepted 2 March 2011
Corresponding author: Departamento de Cardiología, Institut del Tórax, Hospital Clínic, Villarroel 170, 08036 Barcelona, Spain. mroque@clinic.ub.es