This study aimed to evaluate the efficacy and safety of sodium-glucose cotransporter 2 (SGLT2) inhibitors throughout the spectrum of kidney function in patients with heart failure (HF).
MethodsThis meta-analysis included randomized controlled trials comparing SGLT2 inhibitors with placebo in patients with HF stratified by renal function. Literature from inception to June 8, 2024 was searched. The primary outcome was a composite of cardiovascular death or HF events.
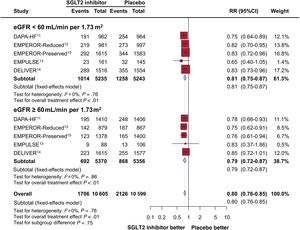
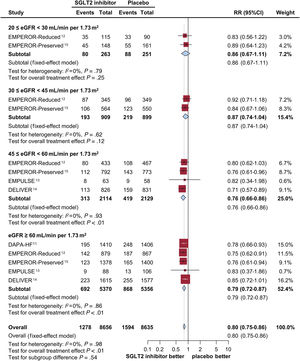
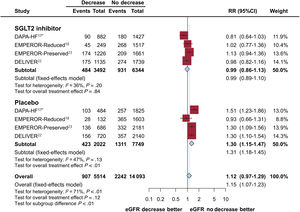
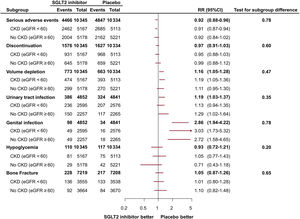
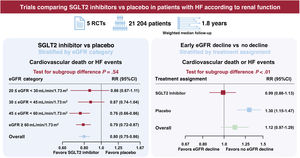
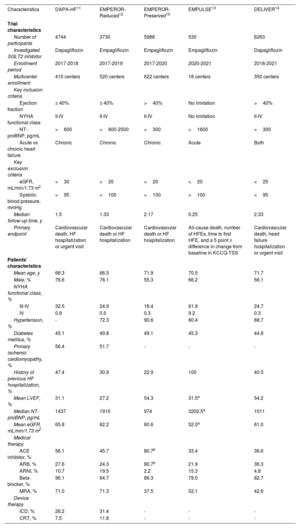
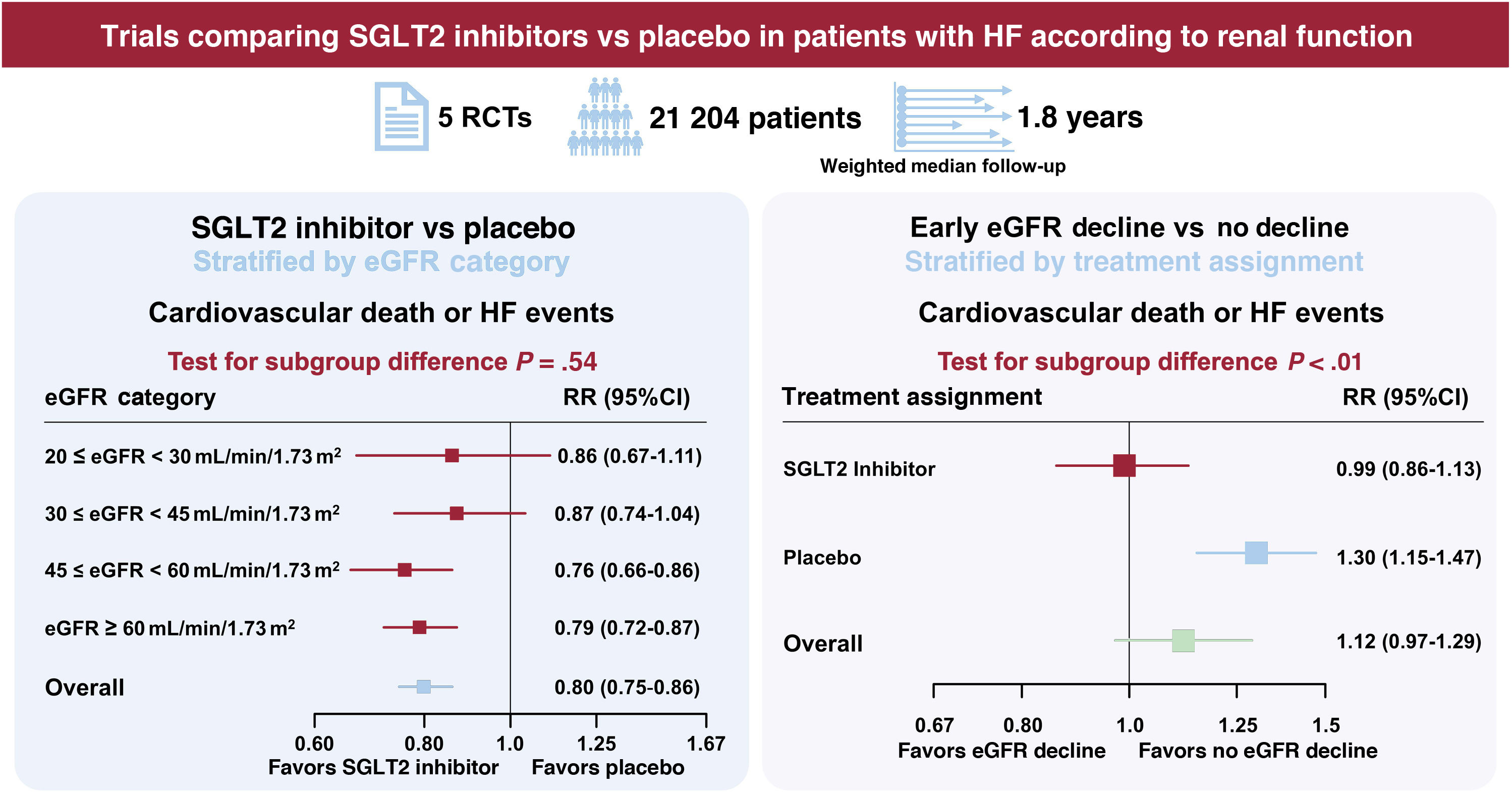
ResultsFive trials were identified, comprising 21 204 patients (10 605 in the SGLT2 inhibitor group and 10 599 in the placebo group) who were randomized and followed up for a weighted median duration of 1.8 years. When patients were classified by estimated glomerular filtration rate (eGFR) of 60mL/min/1.73 m2, SGLT2 inhibitors reduced the risk of the primary outcome irrespective of kidney function (RR, 0.81; 95%CI, 0.75-0.87; P<.01 for eGFR <60mL/min/1.73 m2; RR, 0.79; 95%CI, 0.72-0.87; P<.01 for eGFR≥ 60mL/min/1.73 m2; test for subgroup differences P=.75). The beneficial impact of SGLT2 inhibitors was consistently observed when patients were further subclassified by eGFR values of 20-30, 30-45, 45-60, and >60mL/min/1.73 m2 (test for subgroup differences, P=.54). Early eGFR decline showed a differential impact with increased risk only in the placebo subgroup (RR, 1.30; 95%CI, 1.15-1.47; P<.01), but not in the SGLT2 inhibitor subgroup (RR, 0.99; 95%CI, 0.86-1.13; P=.84) (test for subgroup differences, P<.01).
ConclusionsSGLT2 inhibitor therapy is safe and effective throughout the spectrum of kidney function and regardless of the initial decline in kidney function in patients with chronic HF.
Registered at PROSPERO: CRD42024565218.
Keywords
Identify yourself
Not yet a subscriber to the journal?
Purchase access to the article
By purchasing the article, the PDF of the same can be downloaded
Price: 19,34 €
Phone for incidents
Monday to Friday from 9am to 6pm (GMT+1) except for the months of July and August, which will be from 9am to 3pm