Keywords
INTRODUCTION
Primary angioplasty (PA) has been shown to be more effective than thrombolysis in the treatment of an ST segment elevation acute myocardial infarction (STEMI) as it achieves a final Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow in the infarct-related artery in approximately 90% of cases. Also, in comparison to thrombolysis, it decreases mortality rates, re-infarction, recurrent ischemia, the need for new revascularization, and the incidence of intracranial haemorrhage.1
Although better coronary reperfusion is obtained with PA compared to thrombolysis after an STEMI, endothelial function has not been studied in detail following the ischemia-reperfusion episode in patients treated with PA, despite endothelial dysfunction having been described in experimental models during episodes of ischemiareperfusion after coronary occlusion.2,3 Recently, early endothelial dysfunction in the infarct related artery (IRA) and its recovery 1 year later, has been described in patients with an STEMI treated with thrombolysis. These results suggest a component of stunned endothelium in the first days following the infarct.4 It has also been shown, that the coronary flow reserve improves 6 months after PA compared to that achieved immediately post-angioplasty.5
The aim of the study was to determine whether endothelial function in the IRA after a STEMI depends on the type of reperfusion. We evaluated the degree of endothelial function in the IRA in the first days after a STEMI treated with PA, and compared the data with a similar cohort of patients treated with thrombolysis.
METHODS
Patients
Thirty-seven consecutive patients with an STEMI treated with PA within 6 h of onset were initially included in our study from 2003 to 2004. All patients received aspirin (250 mg, oral) prior to catheterization and a bolus of heparin (100 IU/kg, intravenous) was administered during the procedure. Direct coronary stenting and use of glycoprotein IIb/IIIa receptor inhibitors were left to the discretion of the attending cardiologist. All patients had bare metal stents implanted: 7 Vision® (Guidant Corp., Santa Clara CA, USA); 4 Driver® (Medtronic Inc., Minneapolis, MN, USA); 4 Sonic® (Cordis Corp., Miami Lakes, FL, USA); 2 Liberté® ( Boston Scientific Corp., Natick, MA, USA); and were prescribed aspirin and clopidogrel for at least 1 month following the procedure. Exclusion criteria were: a prior history of myocardial infarction in the same coronary territory; Killip class IV; age >75 years; previous coronary revascularization; insulin-dependent diabetes mellitus; renal insufficiency; severe hypertension; angina that required treatment with vasodilators; and connective tissue disease. Also, the patients needed to provide fully informed consent to undergo a second, elective, catheterization approximately 9 days after the initial revascularization. Other exclusion criteria were angiographic characteristics such as: TIMI flow grade <3 in the IRA; main stem disease; diffuse coronary disease; and the inability to study endothelial function in the IRA.
For comparison, 16 other patients with an STEMI reperfused with thrombolysis between 1997 and 1998, and in whom evaluation of endothelial function using the same protocol was performed also 9 days after infraction, were used as a control group. These patients came from a previous study by our group and had been treated with aspirin (250 mg) and streptokinase (1.5´106 IU) or recombinant tissue-type plasminogen activator (100 mg in an accelerated manner) plus intravenous heparin. All the patients were included consecutively and the exclusion criteria were the same as in the group treated with PA.4
Criteria of Reperfusion
Time-lapse between pain-onset and opening of the IRA were measured in the PA group. Time to resolution of ST elevation of more than 50%, remission of chest pain, number of new Q waves, and reperfusion arrhythmias were recorded in both groups. In the group treated with thrombolysis, the time-lapse between pain onset, implementation of thrombolysis, and pain remission were recorded and time-to-reperfusion was assessed by measuring successive values of creatine kinase-MB fraction (CKMB) and myoglobin, as has been described previously.4
Endothelial Function Study Protocol
The study complied with the declaration of Helsinki, and the hospital's ethics committee approved the protocol. All vasoactive and anticoagulant medication was discontinued at least 48 hours before catheterization. Coronary angiography was planned approximately 9 days after the STEMI, with standard technique via femoral access and 2500 IU of heparin being administered at the start of the procedure. Selective coronary angiograms and left ventriculography were performed, and the administration of nitroglycerin was avoided. On identifying the IRA, additional 2500 IU of heparin were administered and the 2 orthogonal projections that best visualized the trajectory of the artery were selected for analysis. A 2.5/3F infusion catheter (Transit, Cordis®, Miami, FL, USA) was advanced over a guide-wire and placed proximally to the stented region in the PA group, and proximally to the culprit lesion in the thrombolysis group. The guide-wire was then removed to preclude wire-induced coronary spasm. To determine baseline vasomotor response, physiologic saline was infused for 1 minute through the catheter and an angiogram was recorded. To assess endothelium-dependent coronary vasomotor response, serial doses of acetylcholine were selectively infused in the IRA at a rate of 2 mL/min using a precision pump injector (Harvard, Southnatick, MA, USA). The concentrations of acetylcholine prepared were of 1, 10, and 100 µmol/L, and the final blood concentrations of acetylcholine were estimated at 0.01, 0.1, and 1 µmol/L on the assumption that blood flow in the coronary artery was 80 mL/min.6 Angiograms were performed with identical orthogonal views and radiographic characteristics following the infusion of each acetylcholine solution. Because acetylcholine causes endothelium-dependent vessel relaxation in experimental models and in humans, a paradoxical vessel constriction following the infusion of this substance is an indicator of endothelial dysfunction.7 Finally, to assess endothelium-independent vasomotor function, 2 mg of nitroglycerine were administered in bolus through the guiding catheter and angiography was repeated.
Quantitative Coronary Analysis
Quantitative coronary angiography was performed following the saline infusion, at the end of each infusion of acetylcholine and after nitroglycerine. Angiograms were performed in the 2 orthogonal projections that best showed the artery-of-interest. Images were acquired with a digital x-ray system (Siemens, Munich, Germany) at 25 frames/s and used for subsequent computer analyses. End-diastolic frames were used for quantification by means of a fully automated border-detection quantitative coronary analysis that had been validated previously.8 Calibration of the system is based on the dimensions of the guiding catheter without contrast. Mean luminal diameter of the segments distal to the culprit lesion or stent in the studied artery, were averaged for the 2 projections at baseline, after infusion of stepwise doses of acetylcholine and after nitroglycerine. Mean luminal diameter was determined in 2 or 3 segments depending on the site of the culprit lesion (proximal or middle), each of 15 to 20 mm in length, and in relation to anatomical landmarks. The percentage of change in mean luminal diameter after each infusion, relative to baseline, was recorded. All quantitative measures were performed off-line by the same investigator who was blinded with respect to the protocol study. Endothelial dysfunction was defined as a vasoconstriction of the segment beyond the variability of the method of analysis (>4%) at maximal dose of acetylcholine, as has been described previously.4
Statistical Analysis
Qualitative variables are expressed as absolute value and percentage, while quantitative variables are expressed as mean (standard deviation). Comparisons between groups were made with the Student t test for continuous variables and either the c2 test or the Fisher exact test for categorical variables, where appropriate. Comparisons of coronary segment diameters at different acetylcholine doses were made using the analysis of variance for repeated measurements, which is based on a general linear model for repeated measurements, with multiple paired comparisons corrected by the Tukey method, so as to assess the effect of belonging to the treated or control groups. Interactions between group and coronary diameter, and between group and the percentage of changes in coronary diameter after acetylcholine infusion, were assessed. Analyses of residuals (dfbeta and typified dfbeta) were performed. Correlations between quantitative variables were determined with the Pearson correlation coefficient. A P value less than .05 was considered significant. The SPSS software (SPSS Inc; Chicago, IL) was used for all analyses.
RESULTS
PA had been performed in 86 patients over the study period; of these, 37 patients had no exclusion criteria and were initially included in the present study. However, before the study of endothelial function could be performed, 1 died, 1 had a cerebrovascular accident, 1 had gastrointestinal bleeding, 4 had minor complications at the site of vascular access after PA, and 14 chose not to have a repeat catheterization. Endothelial function was assessed in the remaining 16 patients. The group treated with thrombolysis was also composed of 16 patients, of whom 11 (69%) had been treated with tissue plasminogen activator and the other 5 with streptokinase.4 Table 1 shows the baseline characteristics of patients in the 2 treatment groups. There were no statistically significant differences between the groups with respect to age, gender, prevalence of risk factors, time to reperfusion, and extent of the infarct. Due to the fact that the 2 groups had been studied at different points in time, there was a significant difference regarding treatment with statins and beta-blockers. Per protocol, all medication that could influence the assessment of endothelial function was withdrawn 48 hours beforehand, except for treatment with aspirin and clopidogrel in all patients in the PA group and aspirin or ticlopidine in all patients in the thrombolysis group.
Primary Angioplasty
Patients treated with PA received abciximab prior to, or during the procedure in 12 cases (75%). A coronary occlusion with thrombus was found in all patients except in 1 who had thrombus and TIMI grade 2 flow. Two patients (12.5%) had 2 lesions treated and the rest of the patients had only 1 lesion treated. Complete revascularization was achieved in 11 patients (68.8%). A direct stent without pre-dilatation was implanted in 9 cases (56.3%). Mean diameter of the stents used was 3.22 (0.26) mm and mean length was 21.75 (5.69) mm. The phenomenon of no reflow occurred in 2 patients (12.5%) and an image of thrombus remained following the procedure in 3 patients (18.8%). A final TIMI grade 3 flow was achieved in the IRA in all patients.
Study of Endothelial Function
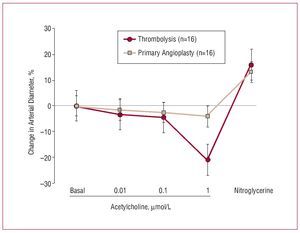
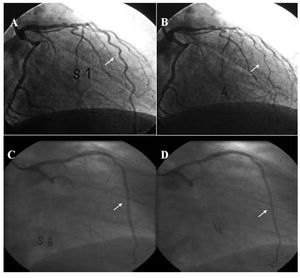
Assessment of endothelial function was conducted within 9 (2) days of the infarct in both study groups. The angiographic characteristics are shown in Table 1. Table 2 shows the diameter of the arteries after intracoronary infusion of acetylcholine and nytroglicerine in both study groups. Intracoronary infusion of acetylcholine produced a dose-dependent vasoconstrictive response, which was indicative of endothelial dysfunction in both study groups. The percent change in arterial diameter after maximal dose of acetylcholine in the PA group was -4 (5%) compared with -20 (21%) in the thrombolysis group (P=.018), indicating a lesser degree of endothelial dysfunction in the IRA after PA than after thrombolysis. Vasodilatation in response to nitroglycerine was observed in both groups (13 [7%] in the PA group vs 16 [3%] in the thrombolysis group; P=non significant), suggesting that non-endothelial-dependent vasodilatation was conserved (Figure 1). Given the difference in age and ejection fraction between both groups, although not significant, we built a multiple regression model that demonstrated that these variables did not confound or interact with the relationship between the intervention group (PA or thrombolysis) and the percent change in coronary diameter at maximum dose of acetylcholine. No significant changes occurred in arterial pressure or heart rate during the course of acetylcholine administration. There were no clinical complications during the procedures. Figure 2 shows an example of endothelial function assessment in both groups.
Figure 1. Percent change in arterial diameter at different doses of acetylcholine in patients treated with PA or thrombolysis after an ST elevation AMI. At the maximum concentration of acetylcholine (ACh) the patients treated with thrombolysis showed a more significant vasoconstriction response in the infarct related artery compared with the ones treated with PA (P=.018). Both groups exhibited a vasodilatation response to nitroglycerine, suggesting that the non-endothelium-dependent vasodilatation is conserved.
Figure 2. Examples of assessment of endothelial function. Angiograms A and B show an example of severe endothelial dysfunction 9 days after thrombolysis, with a marked percent change in arterial diameter at maximal dose of acetylcholine (B), compared with baseline (A). Angiograms C and D show an example of a mild degree of endothelial dysfunction 9 days after primary percutaneous coronary intervention, with a slight percent change in arterial diameter at maximal dose of acetylcholine (D) compared with baseline (C). The arrows indicate the infarct-related artery.
Correlates of Endothelium-Dependent Vasomotor Response
Endothelial dysfunction in the PA group did not correlate with time to reperfusion, use of abciximab, direct stenting, or extent of the infarct. The percent change in arterial diameter at the maximum dose of acetylcholine in the PA group only approached a significant correlation with the concentration of total cholesterol (r=-0.46; P=.07) and low density lipoprotein cholesterol on admission (r=-0.49; P=.056). Conversely, the percent change in arterial diameter at the maximum dose of acetylcholine in the thrombolysis group only correlated with parameters indicative of extent of the infarct: Maximum value of CKMB (r=-0.53; P=.05), area under the curve of CKMB (r=-0.49; P=.05) and the number of new Q waves (r=-0.62; P=.04), as we had previously described.4
DISCUSSION
Our study demonstrates that 9 days after an STEMI, patients treated with PA have a slight endothelial dysfunction in the IRA compared to the more severe endothelial dysfunction present in the IRA of patients treated with thrombolysis.
The endothelium plays a crucial role in the regulation of vascular tone via the release of vasoactive substances, notably nitric oxide, endothelin, prostacyclin, and angiotensin. In addition, endothelial cells are involved in the modulation of platelet activation, leukocyte adhesion, and thrombosis.9 Studies in experimental animals have demonstrated that endothelial nitric oxide bioavailability following a myocardial infarction represents a critical determinant for endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular dysfunction, and survival.10 Endothelial nitric oxide bioavailability depends not only on its production via nitric oxide synthase enzymes but also on preventing its inactivation by free radicals such as the superoxide anion. In the pathophysiology of the ischemia-reperfusion injury after a myocardial infarction, neutrophils have been shown to play a major role in generating free radicals, degranulating products, arachidonic acid metabolites, and platelet activating factors which interact with endothelial cells that induce endothelial injury and neutralization of nitric oxide vasodilator capacity.11
PA is the preferred option in the context of an STEMI.1 The results of our study suggest that less early endothelial dysfunction in the IRA is observed after PA in comparison to a control group of patients treated with thrombolysis. The mechanisms that could explain this different outcome could be: first, an immediate and more permanent reperfusion after PA could imply less ischemia in the IRA, compared to what happens in patients treated with thrombolysis, where the IRA may open and close for a period of time until a stable reperfusion is achieved.12
Second, significant stenosis was absent in the culprit lesion in the PA group at the time of analysis of endothelial function, compared to a residual stenosis of 62% in the thrombolysis group, despite a TIMI grade 3 flow in all patients. The possible mechanism for less endothelial dysfunction in the IRA of the PA group compared to the thrombolysis group could be an increase in the shear stress, which can induce an increase in endothelial nitric oxide synthase phosphorylation.13 Third, the fact that we used metallic stents in comparison with sirolimus eluted stents is associated with less endothelial dysfunction 2 weeks after an STEMI. This could be associated with reduced secretion of vascular endothelial growth factor in the arteries where a sirolimus eluted stent is implanted.14
Fourth, direct stenting was performed in 53% of cases. This strategy may reduce microvascular injury,15 although its effects on endothelial function compared to pre-dilatation are unknown. Fifth, the use of additional medications in the PA group (clopidogrel in all patients and abciximab in 73% of cases) could have been a factor affecting the differences in endothelial function between the 2 groups. Glycoprotein IIb/IIIa receptor blockade could selectively attenuate microvascular endothelial dysfunction following coronary stenting.16 This beneficial effect appears to be due, mainly, to enhanced nitric oxide bioactivity.17 Clopidogrel also increases nitric oxide production in vitro to a greater extent than aspirin.18
Finally, a diminished vasodilator response could be due to alterations of the nitric oxide effector system, such as the blunted formation of cyclic guanosine monophosphate in response to nitroprusside in the smooth muscle cells of the aorta seen after myocardial infarction induction in experimental animal models.19 This last mechanism would appear not to have been implicated in either of our study groups since the vasodilatation response to nitroglycerine was similar in both groups and similar to a control group in a previous study.4
To reduce endothelial dysfunction in the IRA as soon as possible following an STEMI is a reasonable objective that may be better achieved with PA, together with all the concomitant treatments associated with it. Endothelial dysfunction appears to predict progression of atherosclerosis and cardiovascular events in patients with coronary artery disease.20 Also, enhanced endothelial function in response to a pharmacological intervention appears to identify a group of patients who have an improved cardiovascular prognosis.21 In patients who retain patency of the artery following thrombolysis, endothelial dysfunction in the IRA improves dramatically in the year after myocardial infarction,4 suggesting the ability of the human endothelium to recover its function following an episode of ischemia-reperfusion. It seems reasonable to assume that the sooner this recovery occurs, the better.
In the group treated with PA there was no correlation between the grade of endothelial dysfunction and the size of the infarct, time-lapse to PA, or left ventricular ejection fraction at discharge. Use of abciximab or direct stenting did not correlate with endothelial function; albeit the number of patients assessed being small could have affected the outcomes.
Study Limitations
This was a non-randomized study that compared 2 cohorts of consecutive patients over different periods of time in a single centre and the exclusion criteria were very specific such that we studied a highly selected group of patients treated with reperfusion. As discussed above, the difference in treatment between the 2 groups, particularly with regard to antiplatelet agents, and the degree of residual stenosis in the IRA prior to the study of endothelial function, could have had an influence on the degree of endothelial dysfunction. The current use of clopidogrel associated with aspirin and of early coronary angiography in patients reperfused with thrombolysis could imply that the difference in early endothelial function is not as important as was observed in our study. However, all other medications that could have influenced endothelial function had been discontinued 48h before evaluation.
Clinical Implications
The present study provides considerable insight into vascular physiology in patients with an STEMI treated with reperfusion and suggests that the lesser degree of early endothelial dysfunction obtained following PA, compared to thrombolysis, is another argument in favour of PA if performed within a similar time-period as thrombolysis. Of considerable interest is whether a lesser degree of endothelial dysfunction following an infarction could be associated with improved progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival. Early endothelial function in the IRA could be a model to evaluate the efficacy of different mechanical or pharmacological interventions in the acute phase of a myocardial infarction.
CONCLUSIONS
Treatment with PA with a metallic stent in patients with an STEMI predisposes to less early endothelial vasoconstriction in the IRA in the first days post-myocardial infarction, compared to thrombolysis. The differences in residual stenosis of the IRA and in antiplatelet treatment between both groups could partially explain this difference.
ACKNOWLEDGMENTS
We thank the personnel of the Laboratory of Interventional Cardiology for their help and support in the studies of endothelial function.
ABBREVIATIONS
CKMB: creatine kinase MB fraction
IRA: infarct-related artery
PA: primary angioplasty
STEMI: ST-elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
SEE EDITORIAL ON PAGES 797-9
This study was funded, in part, by a grant from the Sociedad Española de Cardiología.
Correspondence:
Dr. J. González-Costello.
Servei de Cardiologia. Hospital Universitari de Bellvitge.Feixa Llarga, s/n. 08907 L'Hospitalet de Llobregat. Barcelona. España. E-mail: jgcostello@hotmail.com
Received November 11, 2007.
Accepted for publication March 13, 2008.