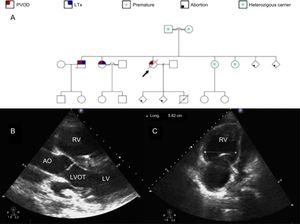
An 18-year-old woman with heritable pulmonary veno-occlusive disease (PVOD) (homozygous carrier of the c.3344C>T(p.P1115L) founder mutation in the EIF2AK4 gene1 [Figure A]) was urgently admitted due to clinical worsening during the last few weeks with presyncope and angina on exertion, third heart sound, an increase in N-terminal pro-B-type natriuretic peptide levels up to 1400 pg/mL, and respiratory insufficiency despite oxygen therapy. Previous treatment with pulmonary vasodilator therapy had to be discontinued due to increased dyspnea and signs of pulmonary edema. Although the patient was advised about contraception, at admission she was pregnant (22nd week of gestation), with 2 previous spontaneous abortions. Although the risk-benefit ratio was explained, she refused to undergo a therapeutic abortion. Despite increasing oxygen supply, intravenous furosemide and inotropes, clinical signs of right heart failure and low cardiac output persisted, while respiratory insufficiency worsened, requiring an increase in fraction of inspired oxygen up to 100%. Transthoracic echocardiogram showed a severely dilated and hypertrophic right ventricle (Figure B-C) with severely impaired function. Gynecological evaluation was performed without signs of fetal distress. The case was discussed with the Heart Team and, since the clinical course was mainly determined by refractory hypoxemia, veno-venous extracorporeal membrane oxygenation (VV-ECMO) support and lung transplantation (LTx) evaluation were decided. Due to the high risk of pregnancy, the patient was again advised about therapeutic abortion, but she decided to continue with the pregnancy, and therefore the case was discussed with the obstetricians and elective cesarean delivery was planned when fetal viability could be guaranteed (at least 24 weeks of gestation). Until delivery, fetal monitoring was performed with obstetric ultrasound and ultrasound for fetal heart rate detection because conventional external monitoring is practically impossible to perform due to the high fetal mobility in those weeks of gestation. Right femoral-right internal jugular vein VV-ECMO was the preferred cannulation configuration with normal decubitus position. After 24hours of clinical stabilization, cesarean delivery was performed (24th week of pregnancy) with no hemorrhagic or thrombotic complications. During the following days, respiratory and hemodynamic improvement allowed inotropic and VV-ECMO withdrawal after 10 days of support. In the meantime, LTx evaluation was performed and after careful assessment of comorbidities, LTx was rejected due to high preformed human leukocyte antigen class I cytotoxic antibodies with an estimated panel reactive antibody higher than 50%, which contraindicates national prioritization on the LTx waiting list. After VV-ECMO removal, the patient showed worsening of respiratory (increased oxygen demand and mechanical ventilation) and hemodynamic parameters (mean pulmonary artery pressure = 53mmHg, cardiac output = 2.4 L/min and right atrial pressure = 30mmHg) without response to medical treatment, finally dying 24hours later. Unfortunately, the newborn also died within the first 24hours after cesarean delivery.
A: familial pedigree. Black arrow identifies our patient. B: transthoracic echocardiogram parasternal long axis view. Severely dilated and hypertrophic RV. C: transthoracic echocardiogram 4-chamber view. Severely dilated RV. AO, aorta; LTx, lung transplantation; LV, left ventricle; LVOT, left ventricular outflow tract; PVOD, pulmonary veno-occlusive disease; RV, right ventricle.
PVOD is a rare cause of pulmonary hypertension (PH), which is part of the special designation (subgroup 1’) within the PH group 1.2 Despite advances in noninvasive diagnosis and knowledge of the genetic basis in the last decade, PVOD remains a rare etiology of PH. There is no approved effective medical treatment and outcomes are poor.3 LTx is the only definitive treatment, with some patients needing extracorporeal circulatory support prior to LTx due to severe respiratory failure. Although ECMO has been increasingly used in the last few years to treat patients with cardiopulmonary failure, there is a lack of evidence about its use in some specific populations, such as pregnant women. Outcomes in this population are limited to small case series and case reports with favorable maternal and fetal survival rates but high hemorrhagic or thrombotic complications.4,5 In a recent comprehensive literature review of all reported cases of ECMO support during pregnancy, Moore et al.5 reported maternal and fetal survival rates of 78% and 65%, respectively. In that review, a total of 45 patients were treated with ECMO during pregnancy and the main indication for ECMO support was severe H1N1 influenza complicated with acute respiratory distress syndrome (73%). VV-ECMO support was used in as many as 91% of the cases reported with a median gestational age of 26.5 weeks and median duration of support of 12.2 days.5 As in nonpregnant adults, the most common ECMO-related complication was bleeding, with major bleeding rates of 57%.6 Interestingly, there were no differences in maternal or fetal mortality rates according to the time of ECMO implantation (second vs third trimester) or the type of ECMO cannulation (VV-ECMO vs venoarterial ECMO).6 However, these results could have been influenced by the small sample size and the likelihood of publication bias. In the present work, we describe what is, to best of our knowledge, the first reported case of cesarean delivery during VV-ECMO support in a pregnant woman with PH due to PVOD.
In conclusion, in experienced centers with a multidisciplinary approach, ECMO support can be used during pregnancy with good maternal and fetal outcomes. Of note, a delivery plan should be prepared for all pregnant patients who receive ECMO, considering both maternal and fetal clinical status. However, the widespread use of ECMO in this population warrants further investigation.
FUNDINGThis work was funded by the Instituto de Salud Carlos III (Ministry of Economy, Industry and Competitiveness) and cofunded by the European Regional Development Fund, through the CIBER in cardiovascular diseases (CB16/11/00502).