Keywords
INTRODUCTION
Chronic heart failure is a clinical syndrome with a poor prognosis in terms of both survival and ongoing symptoms. Heart failure symptoms can seriously compromise patients' well-being, even at rest.1-4 However, there is still no agreement on how to categorize patients with symptomatic heart failure in rigorous and reproducible clinical terms, beyond the New York Heart Association (NYHA) classification.5 Most studies use mortality as the primary outcome variable,6-8 while there have been relatively few studies of the evolution of symptoms and functional status over time,9 or how they are affected by specific interventions.6-8
It might therefore be expected that more detailed studies of health-related quality of life (HRQOL) would have been carried out in heart failure, as such studies could help determine the impact of the disease on health status from the patient's point of view. However, there have been few descriptive studies into patterns of HRQOL impairment in unselected populations of heart failure patients, and there is a particular lack of information on HRQOL impairment in subgroups of clinical interest, determinants of health status, or the way HRQOL evolves over time.10-15 In heart failure populations, HRQOL instruments have principally been used as outcome measures in clinical trials, which makes it difficult to translate the results to clinical practice. Moreover, most clinical trials have used only one disease-specific questionnaire,7,8,16-18 most frequently the Minnesota Living With Heart Failure Questionnaire (MLHFQ). Generic questionnaires have not been used so widely, which makes it difficult to get a broader view of HRQOL impairment in this syndrome.
As a result, knowledge of important aspects of HRQOL in heart failure remains limited. The HF-QoL study was designed in collaboration with 50 hospitals throughout Spain to evaluate the evolution of HRQOL in the first year of follow-up in a reasonably unselected population of patients discharged with heart failure. A further objective was to examine the determinants of mortality in this group of patients.
METHODS
Design
This was a prospective study with a 1 year follow-up and a total of 6 HRQOL evaluations over the study period (before discharge, and at 1, 3, 6, 9, and 12 months after discharge). To ensure that all regions in Spain were represented, clinicians from cardiology and internal medicine departments around the country were invited to participate. Centers did not have to meet any specific clinical or care process characteristics to participate. One or two researchers from each participating center were selected for the study and patients were included between June 2002 and December 2003. Patients meeting inclusion criteria and having none of the exclusion criteria were invited to participate, although they were not necessarily included consecutively. Patients were included in the study if: a) they were admitted to hospital with suspected heart failure, survived admission, and had heart failure as a first or second diagnosis confirmed at discharge, and b) they met European Society of Cardiology standards for diagnosis of heart failure. The latter required a clinical profile compatible with heart failure based on the Framingham criteria and demonstration of cardiac dysfunction using echocardiography, isotopic ventriculography or cardiac catheterization.19 Exclusion criteria were: a) heart failure secondary to an acute reversible cause and no prior diagnosis of heart failure; b) heart failure secondary to severe valvular disease requiring surgery, or chronic cor pulmonale; c) presence of concomitant diseases with an expected survival time of less than 6 months; and d) inability to complete a self-administered HRQOL questionnaire, or presence of any condition or circumstances that would limit follow-up or participation in the study.
In each of the 6 visits, the researchers at participating centers scheduled patients to complete the study questionnaires according to protocol. The clinical questionnaire used at the baseline visit consisted of 52 items designed to collect demographic and clinical data. These included clinical history, etiology, diagnostic test results, and prescribed treatment. Patients also completed the generic SF-36 questionnaire. At follow-up outpatient visits, information was collected on exacerbations, procedures and interventions performed, and any changes in treatment. Data collected were entered in a study-specific web page. At each visit, patients completed two pen-and-paper HRQOL questionnaires (the SF-36 and the MLHFQ) (Hospital Vall d'Hebron, Barcelona) which were submitted to the coordinating center. To obtain data on vital status, telephone calls were made to patients who did not attend the visits.
Patients were informed of the study characteristics and agreed to participate. The study protocol was approved by the Clinical Research Ethics Committee of the coordinating center
Measurement of Health-Related Quality of Life
Patient HRQOL was assessed using a generic instrument (SF-36, version 2) and a disease-specific questionnaire (MLHFQ). The latter was not implemented during the hospitalization period (first evaluation) as it largely refers to activities of daily living.
The 36 items on the generic SF-36 questionnaire measure perceived health status in 8 multi-item scales: physical functioning (PF), role limitations due to physical health problems (RP), bodily pain (BP), general health perceptions (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE), and general mental health (MH). These scores can be summarized in 2 component summary scores of physical (PCS) and mental (MCS) health. Scores range from 0-100, with 100 representing the best perceived quality of life. The instrument has been widely validated for use in Castillian Spanish,20 and standardized data are available for the general Spanish population, making it possible to compare results.
The MLHFQ is a disease-specific questionnaire to measure perceptions of health in patients with heart failure. It contains 21 items and 2 scales (physical and emotional health) which are aggregated into a total score. Scores range from 0-105, with 0 representing the best perceived quality of life.21,22 The MLHFQ has recently been validated for use in the Spanish population.23
The SF-36 (version 2) physical and mental component summary scores were calculated using published algorithms with score imputation for missing data.24 The MLHFQ total score was calculated in the same way, with the algorithm for score imputation being based on the mean score for valid items in the physical and emotional components, and for the total score.25
Statistical Analysis
We calculated the mean (standard deviation) and range to describe continuous variables. Proportions were used for qualitative variables. Patients who completed follow-up were compared with those who did not using the c2 test for categorical variables and the non-parametric Mann-Whitney U test for continuous variables, when normality could not be assumed.
HRQOL scores in subgroups of clinical interest (based on age, sex, functional class) were compared using Student t test or analysis of variance for the SF-36 component summary scores and non-parametric Mann-Whitney or Kruskal-Wallis tests for the MLHFQ scores.
Cumulative survival was analyzed using the Kaplan-Meier method. The same method was used to analyze differences in cumulative survival according to population characteristics. A Cox regression model was used to assess the determinants of mortality at 3 months. Variables included in the model were those demographic, clinical, and treatment variables considered relevant to the model which were found to be statistically significant in bivariate analysis. Variables with a value of P<.1 were introduced in the model using a forward stepwise method. Variables considered for the analysis were age, sex, smoking habit, left heart failure, hypertension, diabetes mellitus, hyperlipidemia, drinking habit, co-morbidity (chronic obstructive pulmonary disease, chronic renal failure, and/or peripheral vascular disease), depressed ejection fraction ( <30 nyha functional class etiology of heart failure and sf-36 pcs mcs scores
The confidence level for the study was set at 95%. The analysis was performed using version 13 of SPSS.
RESULTS
General
A total of 883 patients were enrolled in the study (mean age, 69.2 years; 40.1% female). The sample's clinical characteristics are shown in Table 1. Of particular note were the high proportions of patients with diabetes (41.6%), in NYHA class III or IV at admission (83.1%), or with an ejection fraction <30 23 8
The rate of loss to follow-up was 48.6%, with the highest rate ocurring last between the second and third visits. Completion of follow-up (51.4%) was defined as fulfilling all visits, or until death, during the year. Patients who did not complete follow-up tended to present more serious clinical characteristics, with higher rates of chronic renal failure (18.2% compared to 12.1% in those who did complete follow-up) and a greater likelihood of being in functional class IV at admission (42.4% vs 29.8%). Differences on other prognostic variables were minor (Table 1).
Table 2 indicates the evolution of clinical characteristics in patients who completed each visit. Between the baseline and 1 month visits, there was a significant reduction in the proportion of patients in functional class III or IV, though the proportion of patients in these classes tended to stabilize beyond that point.
Total mortality per year was 22.9%. The highest mortality rate (16%) was recorded in the first 6 months after hospital discharge, and stabilized in the following months.
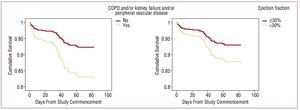
Table 3 presents the baseline predictors of mortality at 3 months. The strongest predictors were age and co-morbidity (chronic obstructive pulmonary disease and/or chronic renal insufficiency and/or peripheral vascular disease), while ejection fraction <30 and a poorer score on the pcs were less strongly associated with mortality cox regression kaplan-meier survival curves are shown in figure 1 p
Figure 1. Survival curves. Kaplan-Meier method.
Baseline Characteristics and Evolution of Health-Related Quality of Life
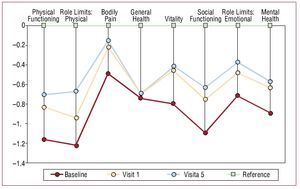
At baseline, SF-36 scores showed significant impairment on all scales. Figure 2 shows the evolution of the 8 scales as the number of standard deviations and in comparison with age- and sex-standardized reference scores for the Spanish general population.20 The most marked impairment occurred in physical health (except pain) and social function. The same figure shows that the improvement observed after one month (with the exception of general health) was maintained over the full study period.
Figure 2. Differences in health-related quality of life (number of standard deviations) between patients included in the study and a Spanish reference population for each of the SF-36 scales and for baseline, and first and last follow-up visits.
Table 2 shows the HRQOL scores at each assessment during the study. In total, 47% of patients for whom there was evidence that they were still alive after one year completed all SF-36 questionnaires and 75.4% completed all MLHFQ questionnaires.
Scores on the SF-36 PSC and MSC at each assessment from hospital discharge to 1 year later also showed initial improvement, with a tendency for scores to then stabilize at values below those of the general population. A similar pattern to that seen on the PSC and MSC was also observed on the physical, mental and total scores of the MLHFQ from the first month after hospital discharge through the first year of follow-up.
HRQOL in Clinical Sub-Groups
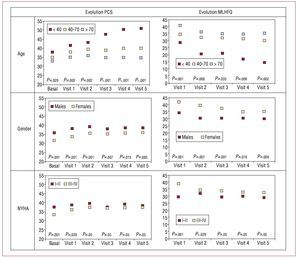
Figure 3 shows the evolution of HRQOL at each visit for clinical subgroups of interest (male/female, age <40 40-70 or 70 years, and NYHA functional class) in terms of scores on the SF-36 PCS and unadjusted MLHFQ total scores. The pattern over time was similar to that observed for the whole population, except in the subgroup of patients under 40 years of age (2% of the sample), to show progressive improvement during follow-up. This was the only group to show statistically significant improvements in HRQOL (PCS and MLHFQ). In the other subgroups, there was a tendency towards stabilization; women, those over 70 years of age, and those in class III/IV followed a course parallel to that of males, patients aged 40-70 years, and those in functional class I/II, respectively, but at lower levels of HRQOL.
Figure 3. Evolution of different health-related quality of life measures by age, sex, and functional class. MLHFQ indicates Minnesota Living with Heart Failure Questionnaire; PCS, physical component summary.
Scores on the MLHFQ and SF-36 showed a similar pattern in those who survived and those who died during follow-up, although values were lower in the latter group (Table 3).
DISCUSSION
In heart failure patients, HRQOL measures have primarily been used as outcome variables in clinical trials,6-8,16-18 although some descriptive studies have been performed in less selected populations and have added to knowledge on the way the disease impacts health status.10-15 Such descriptive studies have also provided evidence of the magnitude of the condition's impact in comparison to other diseases.26 Nevertheless, relatively little is known about how HRQOL evolves over time in patients with the condition.
This study was designed to describe the impairment of HRQOL in conditions of actual clinical practice in a Spanish population of patients hospitalized for heart failure and to investigate patterns of change over 1 year in clinical subgroups of interest. Patients were selected on admission to hospital and only those who agreed to attend follow-up visits were included. However, all patients included were admitted for an exacerbation of heart failure. In terms of representativeness, hospitals from all regions and all levels of care participated. The clinical characteristics of the study population suggested that severity and prognosis were intermediate between the general population affected by heart failure and patients typically included in clinical trials.
Health-related quality of life was assessed using a combination of a generic instrument (the SF-36, version 2) and the disease-specific tool most frequently used in heart failure clinical trials (the MLHFQ).27 Generic instruments provide a more general picture of health status, as they explore a wider range of dimensions and outcomes, and results can be compared with the general population and with patients with other conditions. Disease-specific instruments provide information on dimensions and items of particular interest to a particular patient group and are more sensitive to changes in those patients. On the other hand, results cannot be compared with the general population or with other diseases. The combination of both types of instrument10,26 provides a broader picture of HRQOL impairment, and is particularly appropriate in descriptive studies such as this one.
The results of the baseline assessment of HRQOL showed impairment in most of the scales, except bodily pain, but particularly in physical functioning, role limitations due to physical problems, and social functioning. The magnitude of the impairment at baseline, using both the Spanish general population reference scores28 and values observed in other studies29, may be considered relevant both from a statistical standpoint30,31 (effect sizes of 0.2 to 0.5 represent a small difference; those between 0.5 and 0.8, a moderate difference; and those greater than 0.8, a big difference) and from a clinical and social perspective.32 Taking into account that a score of 50 corresponds to the average score for the general population on the SF-36 summary components, the average baseline PCS (34.1) and MCS (40.1) scores observed here represent significant deterioration according to Cohen's criteria.30,31 If we take the values obtained in other studies in patients with severe heart failure as a reference33, the baseline total MLHFQ score (37.5) observed here can be considered to indicate moderate to severe impairment.
The major limitation of the present study was the substantial loss to follow-up. The comparison between patients who completed follow-up and those who did not indicated that the latter had more serious health problems, so their prognosis and evolution of HRQOL were likely to be poorer. However, both populations were similar with respect to other prognostic variables, so, with the usual caveats, it could be plausible to expect that the pattern of change in HRQOL observed in patients who completed follow-up would apply to the whole group. It should also be noted that the findings from the initial phase of the study (the first 3 months), when the most important loss to follow-up of patients had not yet taken place, would certainly be more valid than those from later stages.
In terms of the evolution of HRQOL over the study period, the most noteworthy aspect was a modest, homogeneous, and clinically relevant improvement in scores by the first visit, followed by a tendency to stabilize without further improvement. The same pattern was observed in both the total population and by subgroup. It was also observed using all three main outcome indicators (PCS, MCS, and MLHFQ) as well as on clinical variables. Among the subgroups, those with poorer clinical characteristics (age >70 years, functional grades III/IV, patients who died during follow-up) showed a similar pattern but with poorer HRQOL overall.
It is plausible to assume that any divergence from this pattern in patients who did not complete follow-up would be negative, due to their poorer clinical condition, ie, they would more likely show deteriorating HRQOL rather than any improvement. For that reason, we believe our results indicate that, except in patients under 40 years of age, the improvement in HRQOL observed after discharge for heart failure in the major clinical subgroups represents the maximum attainable. The best case scenario is that HRQOL remains stable during the first year, though the level of HRQOL will be dependent on a subgroup's specific characteristics. Similar stability was observed after one year in another descriptive study,34 although in that study HRQOL was only evaluated twice, at baseline and on completion of follow-up. This data suggests that, in patients with poor prognosis for survival in the first year, HRQOL will remain generally stable, though at a lower level than in survivors, and will eventually deteriorate before death.
It would be desirable to try to verify the deterioration observed in the latter parts of longer-running clinical trials33 in observational studies, and to identify the determinants of the evolution of HRQOL.
CONCLUSIONS
In summary, this observational study of 883 patients discharged from Spanish hospitals with a diagnosis of heart failure shows a significant initial impairment of overall HRQOL, an impairment that is particularly evident in physical health. This was followed by an improvement in health status, though the values achieved still did not reach those of the general population. The values observed one month after discharge were higher in some clinical subgroups (males, younger patients, those in functional grades I/II, those surviving to one year), but they then remained stable, without further improvement in the first year of follow-up. The same pattern was observed in all sub-groups analyzed, except in patients under 40 years of age, who showed a gradual improvement in HRQOL.
Baseline age, functional status, co-morbidity, and HRQOL were identified as predictors of mortality, results which had previously been observed in other studies.10
IC-QOL STUDY INVESTIGATORS
J. Ariza, J. Fernández, V. López, R. Calvo, P. Bureo, J. Carretero, A. Bayes, D. Gil, C. Ligero, J. Comin, P. Cabero, J. Roure, G. Peñarrojas, M.A. Paz, S. Castro, J. Roca, L. Perdigón, J.A. Ruiz, D. Jiménez, V. Bertomeu, A. Mateu, A. Carrión, S. Martí, A.M. Rubio, J. García, J. Blanquer, J.C. Vargas, C. Pérez, M.A. García, L. Pérez, C. Borasteros, F. Taboada, A. Grande, A.I. Huelmos, J. Bilbao, A. Melero, A. Díaz, J.L. Diago, A. Navarro, J.F. Sotillo, J. Rovira, J.A. Velasco, A. Chaume, F. Atienza, A. Salvador, P. Baello, J. Muñoz, V. Ruiz, M.J. Fombella, J.M. Cerqueiro, E. Freire, J. Jiménez, C. Hidalgo, F. Santolaria, M. Rodríguez, O. Afonso, I. Lekuona, J.A. Alarcón, A. Pérez, J. Marasa, A. del Rio, T. Soriano, E. Roig, I. Vallejo, A. Álvarez, J. Julià, R. Bagà, J. Mesquida, A. Tobaruela, J.M. Lomas, A. Martínez, A. Aguilera, and A.M. Campos.
ABBREVIATIONS
HRQOL: health-related quality of life
MCS: mental component summary
MLHFQ: Minnesota Living With Heart Failure Questionnaire
PCS: physical component summary
SF-36: SF-36 health questionnaire
Study funded by Novartis Farmacéutica.
Correspondence: Dra. N. Soriano.
Unidad de Investigación. EAP Sardenya-IIB Sant Pau. Sardenya, 466. 08025 Barcelona. España.
E-mail: nsoriano@eapsardenya.cat
Received May 14, 2009.
Accepted for publication January 7, 2010.