Keywords
INTRODUCTION
Incidence of cardiogenic shock following open-heart surgery is 2%-6%.1 Pharmacologic support with inotropic drugs and intraaortic balloon pump (IABP) implantation permit 70%-90% of these patients to be weaned from extracorporeal circulation (ECC) although up to 40% die during surgery or in the immediate postoperatory period.2 Ventricular assist devices are indicated in patients with low cardiac index despite the use of inotropics and IABP as a bridge prior to transplantation. They permanently replace cardiac function or help recover myocardial contractility.
Studies of patients with low output following open-heart surgery have shown excellent results with the device in terms of recovery and survival after cardiogenic shock.3-8 The Impella is superior to earlier such devices as delivery and use have been simplified.9 Moreover, few complications have been associated with implantation. Given the good results obtained in patients undergoing surgery, the device has recently begun to be used in percutaneous interventions.10-13
In the current study, we describe our initial experience with Impella ventricular assist devices to prevent and treat low-output syndrome in patients undergoing open-heart surgery and left coronary artery percutaneous angioplasty.
METHODS
We implanted 13 Impella left ventricular assist devices from December 2004 thru December 2006. Seven patients received them for low-output syndrome following open-heart surgery despite inotropic treatment at maximum dosage and IABP. In the remaining 6, the Impella was implanted as an antithrombotic prophylaxis prior to left coronary artery angioplasty. In this group, we measured International Normalized Ratio 4 to 6 weeks prior to implantation and patients underwent echocardiography if values were outside of the range. Median age at implantation for both series was 61 years.
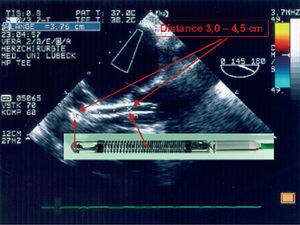
The Impella ventricular assistance device (Impella Cardiosystems AG, Aachen, Germany) is a powerful miniaturized axial flow pump that is inserted backwards in the left ventricle (LV) via the aortic valve. The system is similar to the Hemopump (Medtronic, Inc, Minneapolis, Minn, USA) but incorporates substantial improvements in the console, manner of implantation, delivery, and size. There are 2 devices: 1 for surgical and another for percutaneous interventions and they differ slightly in route of implantation (percutaneous via the femoral artery or open-heart surgery via the ascending aorta, respectively) and maximum pump flow capacity (2.5 or 5.3 L/min, respectively). Correct delivery of the device via the aortic valve is checked through pressure curves and fluoroscopy in angioplasty, and through pressure curves and transesophageal echocardiography in surgical interventions (Figure).
Figure. Transesophageal echocardiogram showing correct delivery of the Impella Recover device via the aortic valve with flow in its interior. The echocardiographic image correlates with the real dimensions of the device.
RESULTS (Table)
Surgical Interventions
In the group undergoing surgical interventions, of 5 patients with ischemic heart disease (1-5 in Table), the Impella device reversed cardiogenic shock in 4, permitting extracorporeal circulation (ECC) to be discontinued and later removal of the device itself. However, neither of the 2 patients with valvular disease (6-7 in Table; aortic stenosis in 1, and mitral stenosis in the other) came out of cardiogenic shock with the ventricular assist device. The median EuroSCORE and Parsonnet scores of patients in this group were 0.88 (range, 0.88-1.70) and 3.1 (range, 1.23-5.43), respectively.
Of the 4 patients with ischemic heart disease in whom removal of the Impella was successful (1-4 in Table), at the time of writing 2 are alive and in functional class I (2 and 3 in Table), with 14- and 12-month follow-ups after implantation. Another 2 (1 and 4 of the Table) died in hospital as a consequence of complications not associated with the device: 1 of nosocomial pneumonia and the other, after a recurrence of postinfarction interventricular communication. The 2 survivors were a 49 year-old man with left coronary artery disease who received complete arterial revascularization with double mammary arteries, and a 53 year-old man with left main coronary artery disease who received inverted saphenous vein grafts.
Of the 2 valvular disease patients, 1 experienced sudden, abundant postsurgical bleeding attributable to the device, following which he died of cardiogenic-hypovolemic shock. This patient received an expanded polytetrafluoroethylene (ePTFE) prosthesis in the ascending aorta. Since this case, we have changed to use a Dacron prosthesis to avoid possible aortic bleeding. No autopsy was performed as the family refused authorization.
Special interest lies in the clinical course of patient number 3 in our series. A 53 year-old man with unstable angina and the equivalent of left and right coronary artery hypoplasia who during median sternotomy presented sudden hypotension and experienced ventricular fibrillation coinciding with ischemic changes in all electrocardiograph leads. We initiated ECC immediately and, with moderate hypothermia and aortic clamping, conducted inverted saphenous vein anastomosis on the mid left anterior descending and first obtuse marginal arteries. In spite of maximum inotropic support and IABP, we were unable to wean the patient from ECC. Echocardiography showed intense akinesia of the interventricular septum and the anterior face of the LV, with hypokinesia of the lateral face and ejection fraction (EF) around 15%. We implanted the Impella Recover device via the ascending aorta and achieved 4.5 L/min, making it possible to discontinue ECC and transfer the stable patient to intensive care with the sternum open. Despite creatine kinase levels having peaked at 15 000 ng/mL, EF improved progressively reaching 45% approximately 70 hours after implantation enabling us to continue to remove the device. The rest of the postoperatory period was without complications. Fourteen months after the event the patient was in New York Heart Association class I and EF had increased to 55%. Parsonnet score of this patient was the highest of those who underwent surgery (5.43). According to our data, this was the first patient in Spain to be discharged after presenting cardiogenic shock following open-heart surgery and being successfully treated with an Impella ventricular device.
Angioplasty
The 6 patients who received the Impella as prophylaxis prior to unprotected left main coronary artery stending were 5 men and 1 woman with moderate or severely depressed EF and at high surgical risk. One had acute pulmonary edema during diagnostic angiography. In all cases, implantation, angioplasty, and removal were without complications. Total time for implantation and testing of the device was <10 min in all cases. None of the patients received an IABP simultaneously.
DISCUSSION
In our population, the Impella proved to be an efficient device both to resolve cardiogenic shock following open-heart surgery and to successfully conduct angioplasty in high-risk patients. In patients undergoing surgery, the Impella resolved cardiogenic shock and was successfully removed in 4 of the 5 patients with ischemia, although 2 of these 4 subsequently died. However, it was inefficient in the 2 patients with valvular disease. In all patients undergoing angioplasty, the procedure was conducted without complications and the device removed at 30 min.
Our results coincide with those reported elsewhere.3-8 Although classically the IABP has been the device of choice for prevention and treatment of cardiogenic shock,14 its use has not been proven to reduce the infarcted area in humans15 and severe complications derived from use have been reported,16 leading some authors to recommend use of the left ventricular device in IABP in high-risk patients.2,17,18 In all patients undergoing surgery, an IABP was implanted prior to the Impella and the 2 devices were used simultaneously.
Similarly, data in the literature also confirm the role of the Impella in high-risk patients who are to undergo angioplasty.10-12.The largest series is that reported by Henriques et al13 in 19 patients, all bad candidates for surgery with LVEF <40% and prior acute myocardial infarction in 74%. Two patients died of causes not associated with the device.
It is evident that one of the limitations of the current study is that we are dealing with a small number of patients. Moreover, the group is heterogeneous and the study was conducted at a single institution. However, the Impella ventricular assist device is easy to implant and has a low rate of complications. It can prove useful in specific patients with low output following open-heart surgery as a bridge prior to heart transplantation and in patients with acute heart failure as in acute mitral failure or myocarditis. Larger studies with more homogeneous patient groups will finally determine its role.
Correspondence: Dr. V. Bautista-Hernández.
Servicio de Cirugía Cardiovascular.
Hospital Universitario Virgen de la Arrixaca. Ctra. Madrid-Cartagena, s/n. 30120. El Palmar. Murcia. España.
E-mail: vbautista_hernandez@hotmail.com
Received October 16, 2006.
Accepted for publication April 12, 2007.