Data have been reported that indicate that cyclosporine may reduce reperfusion injury and the size of myocardial infarctions.1,2 The multicenter CIRCUS trial3 randomized 970 patients with anterior ST-segment elevation myocardial infarction (STEMI), treated by primary percutaneous coronary intervention (PCI) within the first 12hours of symptom onset, and with complete occlusion of the culprit vessel, to receive an intravenous bolus of cyclosporine (2.5mg/kg body weight) or placebo prior to reperfusion of the vessel. The aim was to test the hypothesis that cyclosporine can improve the clinical course and prevent ventricular remodeling in STEMI. The events of the primary end point (a composite of all-cause mortality, heart failure, rehospitalization, or left ventricular remodeling with an increase in left ventricular end-diastolic volume ≥ 15% at 1 year) were recorded in 59.0% and 58.1% of the patients in the cyclosporine and control groups, respectively (odds ratio [OR]=1.04; 95% confidence interval [95%CI], 0.78-1.39; P=.77). Moreover, treatment with cyclosporine did not reduce the incidence of any of the separate components of the primary end point.
The TOTAL trial4 randomized 10 732 STEMI patients treated with primary PCI to a strategy involving systematic aspiration thrombectomy vs conventional PCI, to test the hypothesis that manual thromboaspiration could reduce distal embolization and improve microvascular perfusion. The primary outcome was the composite of cardiac death, recurrent myocardial infarction, cardiogenic shock, or severe heart failure at 180 days, and the safety outcome was stroke at 30 days. Primary outcome events were recorded in 6.9% of the patients in the thrombectomy group vs 7.0% of the conventional PCI group (OR=0.99; 95%CI, 0.85-1.15; P=.86). The rates of cardiac death (thrombectomy vs PCI, 3.1% vs 3.5%; OR=0.90; 95%CI, 0.73-1.12; P=.34) and of the primary outcome plus stent thrombosis or revascularization of the target vessel (9.9% vs 9.8%; OR=1.00; 95%CI, 0.89-1.14; P=.95) were also similar.
The rate of stroke within the first 30 days was higher in the aspiration thrombectomy group (0.7% vs 0.3%; OR=1.13-3.75; P=.02), although the reason for this finding is not clear. Because the same results were obtained in a 1-year follow-up, the investigators in the TOTAL trial do not recommend the systematic use of thrombus aspiration in STEMI,5 although its role in the cases of certain patients and lesions remains to be defined (Figure 1).
The ESTROFA-MI registry,6 involving patients over 75 years of age, collected retrospective data on the antithrombotic therapy administered to STEMI patients undergoing primary PCI. This registry included 2131 patients: 221 (10.3%) treated with bivalirudin, 1374 (64.5%) treated with unfractionated heparin only, and 536 (25.2%) treated with abciximab; the mean ages were 81±5, 81.3±4.8, and 79.8±4.0 years, respectively (P<.001). After a 1-year follow-up, the rates of survival free of myocardial infarction or cardiac death were 85.0%, 80.3%, and 83.1% (P=.03), and repeat revascularization was necessary in 2.5%, 3.0%, and 1.5% of the patients (P=.04), respectively. The incidence of definite or probable stent thrombosis was 2.5%, 2.5%, and 3.1% (P=.2), and that of bleeding > 2 according to the BARC (Bleeding Academic Research Consortium) criteria7 was 0.7%, 1.4%, and 1.0% (P=.8), respectively. On multivariate analysis, none of the strategies proved to be an independent predictor of major cardiac events, although the use of bivalirudin was associated with a lower incidence of cardiac death, without significantly increasing the rate of stent thrombosis. The influence of thrombus aspiration was also analyzed in 2 groups matched for a baseline Thrombolysis In Myocardial Infarction flow grade of 0-1 (560 patients who underwent thrombectomy and 490 who did not), but no significant differences were found in terms of cardiac death, reinfarction, need for repeat revascularization, or stent thrombosis.6
Percutaneous Interventions in Coronary Artery Disease: Vascular Access and Drug TherapyThe MATRIX trial8 randomly assigned more than 8400 patients with acute coronary syndrome (STEMI or non—ST-segment elevation myocardial infarction) scheduled to undergo coronary angiography and PCI to the transradial or transfemoral approach. The coprimary end points at 30 days were a composite of major adverse cardiovascular events (death, infarction, or stroke) and a composite of net adverse clinical events (major adverse cardiovascular events and major bleeding, according to the BARC criteria, unrelated to coronary artery surgery), and the analysis was by intention to treat. In the transradial approach group, 8.8% of the patients had a major adverse cardiovascular event vs 10.3% in the transfemoral approach group (OR=0.85; 95%CI, 0.74-0.99; P=.0307). Major adverse clinical events occurred in 9.8% of the patients in the radial access group and 11.7% of those in the femoral group (OR=0.83; 95%CI, 0.73-0.96; P=.0092). The differences were driven by major bleeding, unrelated to coronary artery surgery, according to the BARC criteria (1.6% vs 2.3%; relative risk [RR]=0.67; 95%CI, 0.49-0.92; P=.013) and all-cause mortality (1.6% vs 2.2%; RR=0.72; 95%CI, 0.53-0.99; P=.045). The conclusions of the study indicate that, in acute coronary syndrome patients treated with PCI, radial access reduces net adverse clinical events compared with femoral access because of the reduction in major bleeding and all-cause mortality.8 In the same study, more than 7200 patients were randomized to receive bivalirudin or unfractionated heparin as antithrombotic therapy during PCI for the purpose of analyzing the events in the 2 groups. There were no significant differences between the 2 agents in terms of the rates of major cardiovascular events (10.3% vs 10.9%; RR=0.94; 95%CI, 0.81-1.09; P=.44), net clinical adverse events (11.2% vs 12.4%; RR=0.89; 95%CI, 0.78-1.03; P=.12), stent thrombosis, or urgent target vessel revascularization.9
The multicenter, randomized RIVER-PCI trial10 tested the hypothesis that anti-ischemic therapy with ranolazine (at a dose of 1000mg twice daily) could improve prognosis in patients with coronary artery disease treated with PCI, but with incomplete revascularization, defined as the presence of 1 or more lesions with ≥ 50% diameter stenosis in a coronary artery with a diameter ≥ 2mm). The primary end point was time to occurrence of an ischemic event or the need for repeat vascularization, and the analysis was by intention to treat. More than 2600 patients were included; after a mean follow-up of 643 days, the primary end point occurred in 26% of the patients assigned to ranolazine and in 28% of those assigned to placebo (OR=0.95; 95%CI, 0.82-1.10; P=.48).
The incidence of repeat revascularization or hospital admission for an ischemic event did not differ significantly between the 2 groups. However, 14.3% of the patients in the ranolazine group discontinued the treatment because of an adverse event vs 10.6% in the placebo group (P=.04).10
The BRAVO-311 was designed to evaluate bivalirudin as an alternative to heparin for anticoagulation in patients undergoing transcatheter aortic valve implantation. A total of 802 patients with severe aortic stenosis who were scheduled to undergo transcatheter aortic valve implantation were randomized to one agent or the other. The primary end points of the study were major bleeding within the first 48hours or prior to discharge and net adverse clinical events at 30 days (the composite of major adverse cardiovascular events and major bleeding). The use of bivalirudin did not result in significantly lower rates of major bleeding at 48hours (6.9% vs 9.0%; RR=077; 95%CI, 0.48-1.23; P=.27) or of net adverse clinical events at 30 days (14.4% vs 16.1%; RR=0.89; 95%CI, 0.64-1.24; P=.50).
It was also found that the rates of major adverse cardiovascular events at 48hours were not significantly different (3.5% vs 4.8%; RR=0.73; 95%CI, 0.37-1.43; P=.35). Although the superiority of bivalirudin over unfractionated heparin was not demonstrated, the criteria for noninferiority were met. The investigators concluded that unfractionated heparin, which is much less costly, should continue to be the agent of choice in interventions of this type, but that bivalirudin could be used in patients who cannot be treated with heparin.11
Drug-eluting StentsThe TUXEDO trial12 randomized 1830 patients with diabetes mellitus and coronary artery disease who were scheduled to undergo PCI to receive a drug-eluting stent (DES) with paclitaxel or everolimus. This was a noninferiority trial, with the primary end point of target vessel failure, which was defined as a composite of cardiac death, myocardial infarction, or the need for target vessel revascularization over a 1-year follow-up period.
At 1 year, the paclitaxel DES did not meet the criterion for noninferiority to everolimus DES with respect to the primary end point of target vessel failure (5.6% vs 2.9%; RR=1.89; 95%CI, 1.20-2.99; P=.38 for noninferiority). The rate of target vessel failure at 1 year was significantly higher in the paclitaxel group (P=.005), as were the rates of stent thrombosis (2.1% vs 0.4%; P=.002), and the need for repeat revascularizations of the vessel (3.4% vs 1.2%; P=.002). Thus, for patients with diabetes mellitus and coronary artery disease treated by PCI, the outcome with everolimus DES was better than the outcome with paclitaxel DES.12
The LEADERS-FREE trial13 included more than 2400 patients with a high bleeding risk, according to specific criteria (age > 75 years, need for chronic oral anticoagulation, anemia, etc.), who were scheduled to undergo PCI; they were randomized to receive a polymer-free biolimus A9-DES or a very similar bare metal stent. All the patients received dual antiplatelet therapy for just 1 month. The primary safety end point was a composite of cardiac death, myocardial infarction, or stent thrombosis. The primary efficacy end point was the need for revascularization of the target lesion. After a 1-year follow-up, the primary safety end point had occurred in 9.4% of the patients in the DES group and in 12.9% of those in the bare metal stent group (OR=0.71; 95%CI, 0.56-0.91; P<.001 for noninferiority and P=.005 for superiority). During this period, repeat lesion revascularization was required in 5.1% of the patients in the DES group and in 9.8% of those in the bare metal stent group (OR=0.50; 95%CI, 0.37-0.69; P<.001). The investigators concluded that, in patients at high risk for bleeding treated by PCI, polymer-free biolimus A9-DES were superior to bare metal stents in terms of both efficacy and safety when dual antiplatelet therapy was administered for 1 month only.
Bioresorbable Vascular ScaffoldThe ABSORB III trial14 was designed to compare the efficacy and safety of the Absorb bioresorbable drug-eluting scaffold vs a cobalt-chromium everolimus-DES. In all, 2008 patients with stable or unstable angina were randomized, in a ratio of 2:1, to receive the bioresorbable device or the everolimus-DES during PCI. The primary end point, studied for noninferiority and for superiority, was target lesion failure (cardiac death, myocardial infarction, or target lesion revascularization) after 1 year. This end point occurred in 7.8% of the Absorb group and in 6.1% of the DES group (95%CI, –0.5 to 3.9; P=.007 para noninferiority and P=.16 for superiority). There were no significant differences between the 2 groups in terms of rates of cardiac death (0.6% and 0.1%, respectively; P=.29), target vessel myocardial infarction (6.0% and 4.6%; P=.18), target lesion revascularization (3.0% and 2.5%; P=.50), or stent thrombosis at 1 year (1.5% and 0.7%; P=.13). The investigators concluded that, for the treatment of noncomplex coronary artery lesions, the bioresorbable drug-eluting device was noninferior to an everolimus-DES with respect to target lesion failure at 1 year.14
ABSORB Japan15 was a multicenter, randomized (in a 2:1 ratio) clinical trial requested by the Japanese regulatory agency for the approval of the use of the Absorb device in Japan. For this purpose, the investigators compared the clinical and angiographic outcomes at 1 year with those obtained in patients who received a cobalt-chromium everolimus-DES, using a noninferiority design. A total of 400 patients were included. The primary end point was target lesion failure (cardiac death, myocardial infarction of the target vessel, and repeat revascularization of the target lesion) at 12 months. The secondary end point was late lumen loss in the treated segment at 13 months. The rate of occurrence of the primary end point was very similar with both the Absorb device and the DES (4.2% vs 3.8%, respectively), that of definite or probable thrombosis was identical (1.5% in each group), and that of the ischemia-driven need for repeat revascularization was similar (1.1% vs 1.5%). The rate of late lumen loss was also very similar (0.13±0.30mm vs 0.12±0.32 mm). The investigators concluded that the clinical and angiographic outcomes were virtually identical in the selected population.
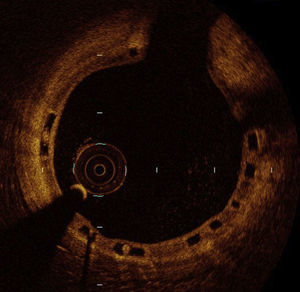
The ABSORB-STEMI TROFI study16 is a multicenter clinical trial that included only STEMI patients treated with primary PCI (n=191), randomized to receive an Absorb bioresorbable drug-eluting device or a cobalt-chromium everolimus-DES. The primary end point was the degree of vascular repair at 6 months, determined by optical coherence tomography (OCT), and based on the presence or absence of intraluminal material and on the apposition and coverage of the device struts (Figure 2). The rate of successful device implantation was high in both groups (96% and 100%, respectively). At 6 months, it was possible to evaluate the primary end point in nearly 90% of the patients, and the healing score was lower in the Absorb group (1.74±2.39 vs 2.80±4.44; P<.001). Angiography showed a significantly greater late lumen loss in the Absorb group (0.20±0.31mm vs 0.08±0.28 mm; P=.01). The rate of adverse clinical events at 6 months was very low (1.1% vs 0.0%), with only 1 case of subacute thrombus. The investigators concluded that their trial confirmed a high and similar degree of vascular repair with both devices, evaluated by the healing score 6 months after primary PCI in STEMI.
Optical coherence tomography image of an Absorb 2 bioresorbable drug-eluting stent months after implantation in a patient with ST-segment elevation acute myocardial infarction. The image shows the homogeneous endothelialization of the struts, without malapposition or intraluminal material.
The aim of the prospective, multicenter BIOSOLVE II trial17 was to evaluate the safety and efficacy of a second-generation, metallic (magnesium) and bioresorbable sirolimus-eluting device in patients with native coronary lesions (reference diameter, 2.2-3.7mm). Angiographic follow-up was scheduled at 6 months, with evaluation by intracoronary imaging in a subgroup of patients. In all, 123 patients were included, and the primary end point was in-segment late lumen loss at 6 months, which was 0.27±0.37mm. The intravascular ultrasound (IVUS) study showed a preserved lumen area at 6 months (6.24±1.15mm2 after PCI vs 6.21±1.22 mm2 at 6 months), with a small mean neointimal area (0.08±0.09mm2). No intraluminal masses were detected with OCT. Target lesion failure was reported in 3% of the patients, and there were no definite or probable thromboses. The investigators concluded that this new drug-eluting, absorbable, metallic device (Figure 3) was safe and effective, and could represent an alternative to bioresorbable polymeric devices for the treatment of obstructive coronary disease.17
INTRACORONARY IMAGING TECHNIQUESThe randomized, multicenter IVUS-XPL trial18 evaluated the possible long-term clinical benefit of IVUS guidance of PCI for DES implantation in long coronary lesions (≥ 28 mm in length) rather than angiography alone. The primary end point was a composite of major cardiac events (cardiac death, myocardial infarction, or target lesion revascularization) 1 year after PCI, analyzed by intention to treat. A total of 1400 patients were included, and 1-year follow-up was completed in 95%. At 1 year, major cardiac events had occurred in 2.9% of the IVUS-guided PCI group and in 5.8% of the angiography-guided group (OR=0.48; 95%CI, 0.28-0.83; P=.007). The difference was due to a lower risk of ischemia-driven target lesion revascularization in patients who underwent IVUS (2.5% vs 5.0%; OR=0.51; 95%CI, 0.28-0.91; P=.02), whereas there were no significant differences between the 2 groups in terms of the rates of cardiac death or target lesion-related myocardial infarction. The only significant difference in the treatment received by each group was the more frequent use of postdilatation of the DES in the IVUS group (76% vs 57%; P<.001).
The ILUMIEN II study19 was designed to determine whether OCT-guided PCI resulted in a degree of stent expansion similar to that achieved with IVUS guidance, since the expansion achieved is considered to be the major predictor of adverse events (thrombosis and restenosis). For this purpose, patients from the ILUMIEN I study20 (OCT-guided PCI and fractional flow reserve) and patients from the ADAPT-DES study21 (IVUS-guided PCI) were included. Stent expansion was studied in 940 patients with covariate-adjusted analysis and in 572 propensity-matched patients.19 In the matched-pair analysis, there were no significant differences in the degree of stent expansion between OCT and IVUS (median, 72.8% vs 70.6%; P=.29). Again, no significant differences were observed after adjustment for baseline differences in the total study population (P=.84). Although the incidences of stent malapposition, tissue protrusion, and edge dissections were higher with OCT, the rates of major malapposition, tissue protrusion, and dissections were similar in the 2 groups. Thus, the investigators concluded that the 2 techniques resulted in similar degrees of stent expansion.20
STRUCTURAL CARDIAC INTERVENTIONAL PROCEDURESTranscatheter Aortic Valve ImplantationThe balloon-expandable SAPIEN 3 transcatheter aortic valve was evaluated in 583 patients (high-risk or inoperable) included in the prospective PARTNER II SAPIEN 3 trial.22 The new valve implements improvements such as low-profile delivery, a smaller stent size, and an external seal to reduce paravalvular regurgitation. The approach was transfemoral in 84% of the patients, transapical in 10%, and transaortic in 6%. The mean patient age was 82.6 years (58% men) and the mean Society of Thoracic Surgeons score was 8.4%. Median 1-year survival was 85.6%, with a rate of cardiac death of 8.1% and a stroke rate of 4.3%. The need for a permanent pacemaker was 16.9% at 1 year. The investigators concluded that these 1-year outcomes indicated that transcatheter aortic valve implantation was the treatment of choice in high-risk or inoperable patients with severe aortic stenosis.
The PARTNER 1 trial23 demonstrated that 1-, 2-, and 3-year mortality was similar with surgical and transcatheter valve replacement in high-risk patients with severe aortic stenosis. The investigators presented the 5-year outcomes of the 699 patients included. The primary outcome of the study was all-cause mortality. At 5 years, the risk of death was 67.8% in the transcatheter replacement group and 62.4% in the surgical replacement group (OR=1.04; 95%CI, 0.86-1.24; P=.76). There were no cases of structural valve deterioration, although moderate or severe aortic regurgitation developed more frequently in the transcatheter group (14% vs 1%; P<.001) and was associated with a higher mortality rate (72.4% among patients with moderate or severe aortic regurgitation vs 56.6% for those with mild or no aortic regurgitation; P=. 003). These data demonstrate that the long-term clinical outcomes of transcatheter and surgical valve replacement in high-risk patients are similar.
Transcatheter Mitral RepairThe German TRAMI (Transcatheter Mitral Valve Interventions) registry24 was designed to evaluate the safety and efficacy of transcatheter mitral interventional procedures involving a clip device. A total of 828 patients were prospectively enrolled (mean age, 76 years; logistic EuroScore, 20%) treated with the MitraClip between August 2010 and July 2013. The 1-year follow-up data are presented for 749 patients (91%): 63.3% were in New York Heart Association functional class I-II (only 11% at baseline) and the 1-year mortality was 20.3%. On multivariate analysis, predictors of mortality were New York Heart Association functional class IV (OR=1.62; 95%CI, 1.10-2.40; P=.02), anemia (OR=2.44; 95%CI, 1.16-5.12; P=.02), previous aortic valve intervention (OR=2.12; 95%CI, 1.32-3.41; P=.002), serum creatinine ≥ 1.5mg/dL (OR=1.77; 95%CI, 1.24-2.54; P=.002), peripheral artery disease (OR=2.12; 95%CI, 1.41-3.20; P=.0003), left ventricular ejection fraction < 30% (OR=1.58; 95%CI, 1.10-2.31; P=.01), severe tricuspid regurgitation (OR=1.84; 95%CI, 1.23-2.77; P=.003), and procedural failure, defined as conversion to surgery, failure of clip placement, or residual severe mitral regurgitation (OR=4.36; P<.0001). The investigators concluded that a high proportion of patients achieved a significant clinical improvement 1 year after MitraClip implantation, and that failed procedures were associated with a higher mortality rate in this series.
INNOVATIONS IN INTERVENTIONAL PROCEDURES IN CONGENITAL HEART DISEASEDuring 2014 and 2015, interesting innovations appeared in the medical literature on transcatheter treatment of congenital cardiac lesions. Patel et al25 reviewed the percutaneous options for replacement of all 4 valves and reflected on the possibility of extending the indications to include congenital valve disease in younger patients without severe comorbidities, but with a high surgical risk (ventricular dysfunction, multiple reinterventions).
In the last few years, stenosis in a bicuspid aortic valve has been considered a contraindication for transcatheter valve prosthesis implantation. However, in 28 patients with bicuspid valves who underwent this procedure, Kochman et al26 reported outcomes similar to those of a control group of 84 patients with aortic stenosis and tricuspid valve, during a 12-month follow-up.
The transjugular approach is used in a few patients who require transcatheter pulmonary valve implantation in the right ventricular outflow tract. Zampi et al27 analyzed a series of 81 patients; among these, the transjugular approach was used in 14 patients when transfemoral access failed. These authors describe the situations in which transjugular access should be considered the approach of choice: younger patients (children younger than 11 years), patients with moderate or severe tricuspid regurgitation, and patients with high right ventricular pressures.
Awad et al28 described the utility of intracardiac echocardiography during transcatheter pulmonary valve replacement. This technique enabled them to satisfactorily establish the gradients and the existence of regurgitation in the right ventricular outflow tract, before and after placement, and to reduce the need for fluoroscopy and pullback pressures across the implanted endoprosthesis.
The use of computed tomography in the follow-up of patients who have undergone transthoracic pulmonary valve replacement is not common in our setting; for this purpose, magnetic resonance is more frequently performed, as it is more effective in evaluating right ventricular function and ventricular end-diastolic and end-systolic volumes. However, thoracic computed tomography may detect the need for reintervention in the case of the Edwards SAPIEN transcatheter pulmonary valve; the loss of the symmetry and circular shape of the device, as well as diameters smaller than expected for each of the available sizes, may be predictors of the need for overexpansion using a balloon or for a valve-in-valve procedure during follow-up, as indicated by Muller et al.29
Although larger series have been described in the literature, Mallula et al30 present one of the few series in which the results of ductal stenting were compared with those of surgical shunt creation in 2 homogeneous groups of patients with pulmonary atresia and intact ventricular septum. The length of hospital stay was 10 days with the ductal stent vs 23 days with the surgical shunt. The only procedure-related death occurred in the shunt group, although, during postdischarge follow-up, reintervention was required in 7 infants with the ductal stent and 2 in the surgical shunt group. Angioplasty in congenital heart disease in the immediate postoperative period has been somewhat controversial. In recent years, a period of more than 4 weeks after the intervention was recommended in order to safely dilate the surgical suture. However, Nicholson et al31 report a total of 91 interventional catheterizations in pulmonary arteries and aortic arch (dilation or stent implantation), carried out without complications within 30 days after surgery.
The need for stent placement in small patients has been met with disapproval by many authors, with the argument that overdilation until the necessary final diameter is achieved is not always possible once the child has stopped growing. However, Gil-Jaurena et al32 report a series of 35 patients in which 43 stents, previously implanted in different sites, were removed (totally or partially) at the time of corrective surgery, without leaving significant residual lesions.
CONFLICTS OF INTERESTNone declared.