Keywords
INTRODUCTION
The association between ischemic mitral regurgitation (secondary or functional)—detected by auscultation, echocardiography, or left ventricular angiography—and poor prognosis1 in relation to morbidity and mortality after myocardial infarction, chronic heart failure, or percutaneous or surgical revascularization is well known.2 The first studies demonstrated that patients with moderate to severe mitral regurgitation (MR) had a greater risk of mortality and worse prognosis.3 Recently, it has been found that even the mildest grades of MR were associated with worse prognosis.4,5 In fact, the Olmsted study reported that MR was associated with a 3-fold increased risk of heart failure, regardless of age, sex, Killip class at admission, and ejection fraction.6 In general, previous studies have focused on ST-elevation myocardial infarction, during the short or medium term, and conducted in Anglo-Saxon populations. Similarly, in a limited series of patients, it has been reported that degenerative MR before infarction is an independent risk factor.7 Previously, our group had studied the influence of functional MR after non-ST-segment elevation myocardial infarction (NSTEMI) during the short term (mean, 425 days).8
Our aim was to confirm the findings of previous series and to assess the long-term implications of ischemic MR after NSTEMI in our context.
METHODS
Study Population
A total of 255 consecutive patients were selected after their admission to the coronary unit of our center for a first NSTEMI between November 2003 and September 2005 and who belonged to the cohort previously described.8 Patients with hypertrophic cardiomyopathy, valvular/subvalvular structural mitral disease, mitral prosthesis, or mechanical complications were excluded from the study group, with the aim of exclusively selecting those with ischemic MR. Patients with a poor ultrasound window that impeded the quantification of MR by echocardiography and the 18 patients who died during admission were also excluded. The 237 remaining patients were discharged in NYHA functional class I or II and these constituted the study group. The diagnosis of NSTEMI was based on the criteria published by the European Society of Cardiology.9 The medical record, laboratory findings, and hospital course were meticulously collected. All patients were prospectively followed up.
Echocardiographic Studies
Before discharge, all patients underwent complete echocardiography, in which mitral valve anatomy and function were specifically studied during a median [interquartile range] of 2 [1-3] days after admission. Doppler echocardiography was performed using a Philips Sonos 5500 with 2.5-3.5 MHz probes. Left atrial and ventricular diameters were measured in the parasternal view on M mode. The ejection fraction was calculated in 2D-mode, in the apical 2- and 4-chamber apical views, using the Simpson biplane method. Myocardial thickening was assessed by dividing the left ventricle into the 16-segment model, following the recommendations of the American Society of Echocardiography.10
Myocardial regurgitation and its grade were assessed using the proximal isovelocity surface area (PISA) method and a nomogram for semiquantitative estimation, as usually conducted in our laboratory. This method, validated and simplified, is in excellent agreement with the angiographic grade of mitral regurgitation.11-13 Thus, MR was quantified into 5 grades (0 = no MR; I = mild; II = mild to moderate; III = moderate; IV = severe). Patients with trivial MR were included in the group without MR. Systolic pulmonary artery pressure was calculated in reference to tricuspid regurgitation.14
Angiographic Studies
Coronary angiography was performed using standard techniques. Significant coronary disease was defined by angiographic stenosis ≥70% in the epicardial coronary arteries and ≥50% in the left main coronary artery. The extent of coronary disease was characterized by 1-, 2-, or 3-vessel disease.15 Catheterization and percutaneous or surgical revascularization were selected according to the criteria of the physicians in charge.
Follow-up and Recorded Events
After discharge, all patients underwent periodic and prospective follow-up. Telephone interviews were conducted when the patient preferred not to attend the checkup. Follow-up was conducted in each patient until contact was lost or until the last follow-up in December 2007. The following events were recorded during follow-up:
- Death, reported in the medical record or by a family member by telephone. The date was recorded and death classified into 2 subgroups: death from cardiovascular causes or sudden death (classified in this way in the death report or by family members during the interview).
- Unstable angina, acute myocardial infarction or heart failure episode that required hospital readmission and were noted in the discharge report as one of the presumed causes of these admissions.
- Major adverse cardiovascular event (MACE): composite of death or unstable angina or myocardial infarction or heart failure.
Each event was recorded only once; for example, after a heart failure event, new episodes were not taken into account in the statistical analysis of MACEs. For all variables, the patient data were censored after the first event.
Statistical Analysis
The SPSS v15 software package for Windows (Illinois, USA) and Microsoft Office 2007 software package (Washington USA) were used. The baseline characteristics of the patients are expressed as mean (standard deviation), continuous variables as median [interquartile range], and categorical variables as an absolute figure (percentage).
Between-group comparisons were performed using Pearson c2 for qualitative variables and Student t test or Mann-Whitney U test for continuous variables, indicated by the dispersion of data. Mitral regurgitation was classified as a dichotomous or categorical variable, depending on the analysis. To evaluate the reliability of the MR grading method used in our laboratory and reported in this paper, observer concordance (ING) and between-observer concordance (ING-LPI) were calculated in 30 studies using the kappa index. Long-term survival curves of the different groups were obtained using the Kaplan-Meier method and comparisons were obtained using the log-rank test. The Cox proportional hazard regression model was used to analyze and select the variables independently associated with the appearance of long-term events. An excessive number of variables in the multivariate analysis was avoided by reducing their number using a prespecified model that included those known to be associated with prognosis. Thus, age (quantitative), diabetes mellitus, and hypertension (present in discharge reports), kidney failure (creatinine clearance £60 mL/h according to the Cockroft-Gault formula16), LVEF, peak troponin level (quantitative), previous revascularization, current multivessel disease (2 or more vessels), atrial fibrillation, ventricular wall motion abnormalities (present), and MR (qualitative and quantitative) were included as covariates in the final models and several clinical events as dependent variables. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated by backwards stepwise regression analysis (Wald). The last follow-up was conducted on December 28, 2007. The null hypothesis was rejected—no statistically significant differences—using a 2-tailed P value <.05 as cutoff.
RESULTS
Epidemiological Characteristics
The mean age of the patients was 66.19 (12.92) years; 175 patients (73.8%) were men. The incidence of MR (grade ≥I/IV) was 40.08% (95 patients; 73 men). The distribution of MR according to its severity was as follows: grade I in 71 patients (30.1%), grade II in 15 (6.4%), grade III in 6 (2.5%), and grade IV in 3 (1.3%). In this regard, observer (k=0.91) and between-observer (k=0.84) concordance were excellent. There were no statistically significant differences in the distribution of MR grade by sex (P=.392). Table 1 shows the initial characteristics of the patients according to whether MR was recorded in the first echocardiogram or not.
When compared, it can be seen that mean age, incidence of diabetes mellitus, and the percentage of patients with diagnosed previous coronary disease (previously revascularization) were significantly higher in the MR group than non-MR group. Although statistical significance was not reached, there was a greater percentage of hypertension and of kidney failure in the MR group. Medical treatment at discharge was similar in both groups, including beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor antagonists (ARA-II), diuretics, aldosterone blockers, and nitrates.
Initial Echocardiographic Parameters
Analysis of ventricular geometry showed that left ventricular volumes were not significantly different (Table 2). The size of the left atrium was slightly greater in the MR group (39.5 mm vs 42.2 mm; P=.014). The grade of mitral tenting was greater in the MR group. Left ventricular systolic function was decreased in the MR group (LVEF, 51.03 [15.57] vs 59.57 [14.1] in the MR group and non-MR group, respectively; P<.001). The incidence of wall motion abnormalities was greater in the group with significant MR. No significant differences were found in wall thicknesses or transmitral Doppler ultrasound measurements, including E waves, A waves and E/A waves, and systolic pulmonary arterial pressure.
Coronary Anatomy
At admission, 212 (89.45%) patients underwent catheterization (Table 1). The patients with MR presented more extensive coronary disease (P=.003) and there was a greater rate of anterior descending artery disease (P=.063). On the other hand, 134 (56.54%) patients underwent myocardial revascularization during the same admission. The total percentages of revascularization and success (TIMI III) were similar (P=.592). In this series, no patient underwent a combined surgical procedure of revascularization with mitral valve repair or replacement. In addition, no patient underwent valve repair alone, either because there was no indication or because of patient preferences.
Long-Term Follow-up
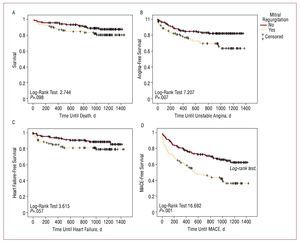
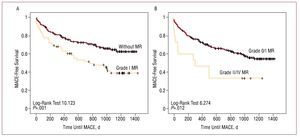
The follow-up period was similar in both groups (P=.608), with a median of 1011 [717.5-1206] days. A total of 10 patients were followed-up for less than 3 months because they died during this period. Recorded clinical events are shown in Table 3. Greater mortality was observed during follow-up in the MR group (P=.106), almost reaching statistical significance when only deaths from cardiovascular causes were considered (P=.056). Admissions for heart failure, although more numerous in the MR group, did not reach statistical significance (10.6% vs 17.9%; P=.106). Readmissions for diagnosed unstable angina in the MR group were more frequent (P=.015). No differences were observed in readmissions for acute myocardial infarction, with or without Q-wave. Finally, regarding composite events (MACE), a greater incidence was found in the MR group (57.9% vs 33.1%; P<.001). All the data previously described are given as raw values. Kaplan-Meier curves show the long-term association of death, unstable angina, heart failure, or MACE with the presence of MR (Figure 1). The following variables were included in all the multivariate models (Cox): age, diabetes mellitus, hypertension, kidney failure, previous myocardial revascularization, LVEF, peak troponin I levels, multivessel disease (2 or more vessels), atrial fibrillation, and ventricular wall motion abnormalities (present). Table 4 shows the details of the multivariate analyses, and only includes the factors that were found to be associated with the appearance of the event under study. Diabetes mellitus was the only independent predictor of poor prognosis in each and all of the outcome variables studied. Age, previous revascularization, troponin I levels, multivessel disease, and MR were independent predictors of some or several of the 4 prognostic variables studied. When MR grade during admission was introduced instead of its dichotomous presence or absence, MR was statistically significantly associated with the development of heart failure (HR=1.646; 95% CI, 1.047-2.588; P=.031) and a composite MACE event (HR=1.673; 95% CI, 1.279-2.189; P<.001). Subsequently, event-free survival analysis using Kaplan-Meier curves and the log-rank test were used to specifically compare long-term prognosis in patients without MR and those with mild MR only (I/IV), and demonstrated that even a mild grade of regurgitation was associated with prognosis (MACE, P=.001) (Figure 2). The results of the multivariate analysis are very similar to those of the general model (Table 4) for MACE. The same analysis was performed by dividing the patients into 2 groups, 1 group without MR or with MR grade I, and the other group of patients with higher grades of MR (≥II), leading to a value of P=.012 (Figure 2). The other data obtained by these analyses were similar to those of the main group, thus they are not described in detail.
Figure 1.Actuarial Kaplan-Meier survival curves. A: death during follow-up in relation to the presence or absence of mitral regurgitation after a first NSTEMI event. B: hospital readmission during follow-up due to unstable angina in relation to the presence or absence of mitral regurgitation. C: hospital readmission during follow-up due to heart failure in relation to the presence or absence of mitral regurgitation. D: death or hospital readmission (MACE) during the follow-up in relation to the presence or absence of mitral regurgitation after a first NSTEMI event. Between-group comparisons were performed using the log-rank test.
Figure 2.Actuarial Kaplan-Meier survival curves. The mitral regurgitation (MR) group exclusively included patients with grade I MR. A: death or hospital readmission (MACE) during follow-up comparing patients without MR and patient with grade I MR after a first NSTEMI event. B: death or hospital readmission (MACE) during follow-up, comparing patients without MR or with grade I MR and patients with grade II-IV MR. Between-group comparisons were performed using the log-rank test.
DISCUSSION
This study is one of the first to investigate the prognostic significance of long-term ischemic MR in our context, specifically after a first episode of NSTEMI. Data were analyzed from patients who died or who had cardiovascular complications of sufficient clinical severity to require re-hospitalization. Although the negative short-term prognostic significance has already been reported,8,17 the results of the present study— obtained approximately 3 years after the index infarction—confirm the previous data. It is well known that MR alone is an independent factor of poor prognosis,5-8,18-25 and when associated with a myocardial infarction prognosis is even worse.5,7,8,19,25-27 In our series, a higher rate of cardiovascular events was observed, including cardiovascular death, during follow-up in patients in whom ischemic MR was detected in the first echocardiogram during the acute phase of NSTEMI. The study population was basically homogeneous, with a certain tendency in the MR group to be older and diabetic, with greater multivessel disease, but with fewer smokers. There were no significant differences in size of the infarction, as measured by creatine kinase and troponin I levels, in spite of which LVEF was significantly worse in the MR group. Nevertheless, when Cox multivariate analysis was conducted for composite events, MR was found to be one of the independent predictors of poor prognosis.
The physiopathological mechanism of ischemic MR is still under discussion, although the current preference is to explain it by a myocardial abnormality rather than by a valvular abnormality in itself,2,28 which fits with the fact that the patients in the IM cohort were older and had more diseased vessels, greater anterior descending artery disease, decreased LVEF, more wall motion abnormalities and a greater degree of tenting. The greater number of diseased vessels in the MR group, in addition to being associated with valvular heart disease, could have had an effect on the increased number of ischemic events, such as unstable angina, during follow-up.
Another uncertain issue is the best time to assess MR: immediately after infarction, weekly, or monthly, taking into account potential reversible myocardial dysfunction (stunning or hibernation). Given that the different studies use different time ranges, we chose to perform echocardiography approximately 48 h after infarction, which is the timing typically used in our center. Regarding the temporal aspect, there were no differences between the different groups that could have biased assessment.
Even though the statistical analysis initially hinted at a greater trend toward more events (death, cardiovascular death, angina, or heart failure) in the MR group, Cox analysis did not include the variable MR in the last step of the model regarding heart failure or death. This could be perhaps explained by the study's occasional lack of power to detect differences. The published works of Grigioni et al29 have already described the role of ischemic MR in the development of heart failure after infarction, although they included patients with Q-wave infarction. This association between MR and heart failure has been regularly and repeatedly demonstrated by many authors in recent years.6,18,28,29 Our results are also consistent with those of other published works, and in particular with those of the Survival and Left Ventricular Enlargement Study. Although the authors could not establish a definitive conclusion in their study either, the data displayed a strong trend that indicated an association between ischemic MR after an acute coronary syndrome and the onset of heart failure.4 Similarly, it seems to follow from the data available that the association between poor prognosis and MR was not only presented by patients with the most severe regurgitations or in the total group, but also negatively influenced the patients with milder grades.
The statistical analysis used aimed at ensuring that the differences found in the baseline characteristics of the patients in the different groups did not interfere with the validity of the results regarding the prognostic implications of these in relation to MR.
Although the study was not designed to evaluate this issue, a separate analysis was performed to assess patients with LVEF >45% and £45%. The results obtained from the patients with an LVEF >45% (n=185) showed a similar and statistically significant trend (log-rank test for MACE, P=.002), which was not reached in the group with depressed LVEF (log-rank test for MACE, P=.185). These findings should be viewed with caution, mainly because of the limited number of patients included in each group, especially in the group of patients with an LVEF <45%, since the statistical power may have been insufficient to detect the differences in the total cohort.
Diastolic function was not specifically explored, although some key parameters were analyzed in its study, such as left atrial diameter and transmitral peak E and A velocities. The E/A ratio did not indicate statistically significant differences between the 2 groups. Thus, until more interpretable data become available in this regard, we prefer not to conduct a more extensive study on diastolic function.
Limitations
In this study, we were not able to distinguish between patients with MR prior to infarction and MR that occurred during or after infarction. Previous MR has recently been described as an additional negative prognostic factor.7 Obviously, it is very difficult to obtain echocardiographic data from patients before the index infarction. However, in many of the patients with severe MR, and given the mean age of the patients studied, in theory it is logical that these problems would have already been detected. Furthermore, the atria were not very dilatated in either of the 2 groups, and although some left atria in the MR group were slightly larger (42.2 mm and 39.5 mm), this seems both reasonable and congruent with new heart valve disease and with the distensibility of the atrium itself. Thus, and given the characteristics of the published studies, we consider this limitation to be of little relevance. Patients were treated according to the norms followed in our institution in recent years, and depended on the preferences of the physician responsible for the patient and on those of the patient. Although being a topic of interest, treatment during follow-up was not analyzed, due to the clear lack of homogeneity and the frequent changes that occurred (as decided by the multiple physicians responsible for the patients) during follow-up. Thus, we consider that the results of the study may more closely reflect the conditions of daily clinical practice. The epidemiological characteristics indicate that there was a much higher percentage of men than women in all the groups studied (<30%). This fact, although matching some of the published series on infarction and NSTEMI, which also included more men, could hinder the generalization of our results to the female population.
CONCLUSIONS
Ischemic MR is frequent in patients after an NSTEMI. This type of valvular heart disease combines with other negative factors to yield poor long-term prognosis, and this is the case even in the mildest grades of MR. Thus, current MR should be carefully assessed after an NSTEMI, and probably requires even more meticulous treatment and stricter follow-up than usual.
ABBREVIATIONS
LVEF: left ventricular ejection fraction
MACE: major adverse cardiac event consisting of death or unstable angina or myocardial infarction or heart failure
MR: mitral regurgitation
NSTEMI: non-ST-segment elevation acute myocardial infarction
Correspondence: Dr. I.J. Núñez-Gil.
Avda. Profesor Martín Lagos, s/n. 28040 Madrid. España.
E-mail: ibnsky@yahoo.es
Received November 25, 2008.
Accepted for publication July 7, 2009.