The optimal chronic antithrombotic regimen for patients with atrial fibrillation undergoing transcatheter aortic valve implantation (TAVI) remains uncertain. Our aim was to compare the incidence of late bleeding events between patients on direct oral anticoagulants (DOACs) and those on vitamin-K antagonists (VKA).
MethodsThis single-center observational study included TAVI patients requiring oral anticoagulation at discharge between 2015 and 2021. The primary endpoint was any clinically significant bleeding event. Secondary endpoints were stroke, heart failure, and all-cause mortality.
ResultsA total of 702 TAVI procedures were performed, with 297 patients requiring oral anticoagulation at discharge. Among them, 206 (69.4%) received VKA and 91 (30.6%) received DOAC. Baseline clinical, procedural and in-hospital characteristics did not significantly differ between groups, except for better renal function among DOAC patients. The median length of follow-up was 2.8 years. The risk of bleeding events was higher in patients receiving DOACs than in those receiving VKA (HR, 2.27; 95%CI, 1.21-4.26; incidence of 9.7 and 4.2 events per 100 patient-years of follow-up for DOAC and VKA patients, respectively). There were no statistically significant differences in the rates of stroke (HR, 1.28; 95%CI, 0.4-4.3), heart failure hospitalization (HR, 0.92; 95%CI, 0.46-1.86), or all-cause mortality (HR, 1.02; 95%CI, 0.68-1.55).
ConclusionsIn older patients undergoing TAVI and receiving anticoagulant therapy for atrial fibrillation, the use of DOAC was associated with a higher risk of late bleeding events than VKA.
Keywords
Transcatheter aortic valve implantation (TAVI) is an increasingly common treatment for patients with severe symptomatic aortic stenosis. Its role has changed over time. Due to growing evidence from various clinical trials in patients with low and moderate risk1–3 and technical refinement of a less invasive procedure than cardiac surgery,4,5 TAVI is currently the technique of choice in the management of severe aortic stenosis in patients older than 75 years.6
However, controversy persists regarding the antithrombotic regimen after TAVI. The POPular TAVI trial found an increase in bleeding events with dual antiplatelet therapy but no evidence of differences in ischemic events or death vs single antiplatelet therapy with aspirin.7 Nonetheless, the optimal chronic anticoagulation option in patients with atrial fibrillation (AF) has yet to be established. Several pivotal clinical trials have shown that direct oral anticoagulants (DOACs) are a safe and effective alternative to anticoagulation with vitamin K antagonists (VKAs).8 However, the extrapolation of these results to patients with a biological aortic prosthesis and, specifically, to those undergoing TAVI is controversial. In addition, the ENVISAGE-TAVI (Edoxaban Versus Vitamin K Antagonist for Atrial Fibrillation After TAVI) trial found an increased incidence of major bleeding with edoxaban vs VKAs in TAVI patients receiving anticoagulant therapy for AF.9
The aim of the present study was to assess the onset of late bleeding events (after discharge) in patients undergoing TAVI who require anticoagulation for AF by comparing patients anticoagulated with DOACs or VKAs.
METHODSStudy design and populationThis single-center prospective observational study enrolled patients who underwent TAVI in our center between January 2015 and December 2021. This period was chosen to include patients who received latest-generation prostheses and to ensure that treatment with DOACs for the prevention of AF-related stroke was available and widespread in Spain. We excluded patients with valvular AF (with a mechanical heart valve prosthesis or significant mitral stenosis) and patients receiving oral anticoagulation for other reasons (eg, previous pulmonary embolism without history of AF).
The cohort was subdivided into patients receiving DOACs at discharge after TAVI and those receiving VKAs. The type of anticoagulant therapy was at the discretion of the treating physician. In VKA-treated patients, VKA dose and blood concentration were adequately controlled by ensuring a time in therapeutic range > 65%. During the hospital stay before the TAVI, all patients received low-molecular-weight heparin and none were treated with DOACs or VKAs, in accordance with the internal protocol of the center.
The study adhered to the recommendations of the Declaration of Helsinki. The local Health Care Ethics Committee approved the data collection and study protocol. All patients signed informed consent for the procedure and data collection.
Study variablesBaseline, procedural, in-hospital, and postdischarge data were prospectively collected in a specific database for TAVI.
Primary study endpointThe primary endpoint was any clinically significant bleeding event. Bleeding events were classified according to the second and third consensus documents of the Valve Academic Research Consortium (VARC).10,11 Secondary endpoints were stroke, heart failure hospitalization, and all-cause death. A subanalysis was performed to examine major bleeding events categorized as VARC 3 or 4.
Follow-upAfter TAVI, systematic in-person outpatient follow-up was performed at 3 months, 1 year, and annually thereafter. All relevant clinical events during follow-up were prospectively recorded in a dedicated database. Patients were asked about study events, and patients’ medical records were searched for these events. The loss of relevant events was minimized by complete access to each patient's medical history (in all health care centers, both hospital-based and primary) in the autonomous community where the study was performed.
Statistical analysisContinuous variables are presented as mean ± standard deviation, and categorical variables as absolute number and percentage. Comparisons were performed using a t-test for quantitative variables with a normal distribution and using the Mann-Whitney U test for variables with a nonnormal distribution. Categorical variables were compared using a chi-square test or Fisher test.
A Cox regression model was used with hazard ratios (HRs) and their 95% confidence intervals (95%CIs) to compare rates of clinical outcomes by treatment group (DOAC vs VKA). A multivariable model was built with adjustment for age and sex and for baseline variables showing significant differences between groups. The Kaplan-Meier method was used to display the cumulative incidence curves of the study endpoints while the log-rank test was used to analyze the statistical significance. A P value <.05 was considered significant in all analyses performed. STATA version 14.0 (STATA Corp, United States) was used for the statistical analysis.
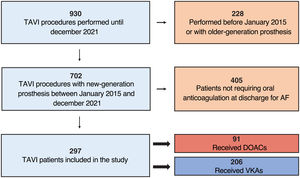
RESULTSOf 702 patients who underwent TAVI between January 2015 and December 2021, 297 required oral anticoagulation at discharge for AF. Of these, 206 (69.4%) received anticoagulant therapy with VKAs and 91 (30.6%) with DOACs. The study flow chart is shown in figure 1.
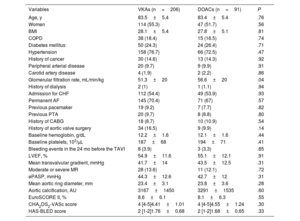
The mean age was 83.5 ± 5.5 years and 161 patients (54.2%) were women. Table 1 shows patients’ baseline characteristics by anticoagulation group at discharge (VKAs vs DOACs). No significant differences were found between the groups in terms of age, sex, body mass index, and the main cardiovascular comorbidities. There was a tendency for better baseline renal function in the patients receiving DOACs vs VKAs (estimated glomerular filtration rate, 56.6 ± 19mL/kg/min vs 51.3 ± 20mL/kg/min; P = .04). There were no significant differences between the groups in baseline hemoglobin (12.1 ± 1.6 vs 12.2 ± 1.6g/dL; P=.44) or platelets (194±71 vs 187±68 × 103 units/μL; P=.41). According to the EuroSCORE II, the cohort showed moderate-high risk (8.4%±6.2%) with no significant differences between the groups (8.6%±6.1% with VKAs vs 8.1%±6.3% with DOACs; P=.55).
Baseline characteristics of the 2 groups
| Variables | VKAs (n=206) | DOACs (n=91) | P |
|---|---|---|---|
| Age, y | 83.5±5.4 | 83.4±5.4 | .76 |
| Women | 114 (55.3) | 47 (51.7) | .56 |
| BMI | 28.1±5.4 | 27.8±5.1 | .81 |
| COPD | 38 (18.4) | 15 (16.5) | .74 |
| Diabetes mellitus | 50 (24.3) | 24 (26.4) | .71 |
| Hypertension | 158 (76.7) | 66 (72.5) | .47 |
| History of cancer | 30 (14.6) | 13 (14.3) | .92 |
| Peripheral arterial disease | 20 (9.7) | 9 (9.9) | .91 |
| Carotid artery disease | 4 (1.9) | 2 (2.2) | .86 |
| Glomerular filtration rate, mL/min/kg | 51.3±20 | 56.6±20 | .04 |
| History of dialysis | 2 (1) | 1 (1.1) | .94 |
| Admission for CHF | 112 (54.4) | 49 (53.9) | .93 |
| Permanent AF | 145 (70.4) | 71 (67) | .57 |
| Previous pacemaker | 19 (9.2) | 7 (7.7) | .82 |
| Previous PTA | 20 (9.7) | 8 (8.8) | .80 |
| History of CABG | 18 (8.7) | 10 (10.9) | .54 |
| History of aortic valve surgery | 34 (16.5) | 9 (9.9) | .14 |
| Baseline hemoglobin, g/dL | 12.2±1.6 | 12.1±1.6 | .44 |
| Baseline platelets, 103/μL | 187±68 | 194±71 | .41 |
| Bleeding events in the 24 mo before the TAVI | 8 (3.9) | 3 (3.3) | .85 |
| LVEF, % | 54.9±11.6 | 55.1±12.1 | .91 |
| Mean transvalvular gradient, mmHg | 41.7±14 | 43.5±12.5 | .31 |
| Moderate or severe MR | 28 (13.6) | 11 (12.1) | .72 |
| ePASP, mmHg | 44.3±12.6 | 42.7±12 | .31 |
| Mean aortic ring diameter, mm | 23.4±3.1 | 23.8±3.6 | .28 |
| Aortic calcification, AU | 3167±1450 | 3291±1535 | .60 |
| EuroSCORE II, % | 8.6±6.1 | 8.1±6.3 | .55 |
| CHA2DS2-VASc score | 4 [4-5]4.41±1.01 | 4 [4-5]4.55±1.24 | .30 |
| HAS-BLED score | 2 [1-2]1.76±0.68 | 2 [1-2]1.68±0.65 | .33 |
AF, atrial fibrillation; CABG, coronary artery bypass surgery; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DOACs, direct oral anticoagulants; ePASP, estimated pulmonary artery systolic pressure; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; PTA, percutaneous transluminal angioplasty; VKAs, vitamin K antagonists.
*Comparisons for CHA2DS2-VASc and HAS-BLED were performed using the Mann-Whitney U test.
Values represent No. (%), mean±standard deviation, or median [interquartile range].
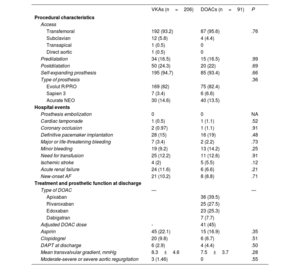
There were no significant differences in baseline echocardiographic characteristics or in aortic valve dimensions or calcification (table 1). Procedural data are reported in table 2. Most implants were performed via transfemoral access (94%) and with a self-expanding prosthesis (94.3%) and there were no significant differences in implant-related variables between the groups.
Procedural and hospital stay characteristics in the 2 groups
| VKAs (n=206) | DOACs (n=91) | P | |
|---|---|---|---|
| Procedural characteristics | |||
| Access | |||
| Transfemoral | 192 (93.2) | 87 (95.6) | .76 |
| Subclavian | 12 (5.8) | 4 (4.4) | |
| Transapical | 1 (0.5) | 0 | |
| Direct aortic | 1 (0.5) | 0 | |
| Predilatation | 34 (16.5) | 15 (16.5) | .99 |
| Postdilatation | 50 (24.3) | 20 (22) | .69 |
| Self-expanding prosthesis | 195 (94.7) | 85 (93.4) | .66 |
| Type of prosthesis | .36 | ||
| Evolut R/PRO | 169 (82) | 75 (82.4) | |
| Sapien 3 | 7 (3.4) | 6 (6.6) | |
| Acurate NEO | 30 (14.6) | 40 (13.5) | |
| Hospital events | |||
| Prosthesis embolization | 0 | 0 | NA |
| Cardiac tamponade | 1 (0.5) | 1 (1.1) | .52 |
| Coronary occlusion | 2 (0.97) | 1 (1.1) | .91 |
| Definitive pacemaker implantation | 28 (15) | 16 (19) | .48 |
| Major or life-threatening bleeding | 7 (3.4) | 2 (2.2) | .73 |
| Minor bleeding | 19 (9.2) | 13 (14.2) | .25 |
| Need for transfusion | 25 (12.2) | 11 (12.6) | .91 |
| Ischemic stroke | 4 (2) | 5 (5.5) | .12 |
| Acute renal failure | 24 (11.6) | 6 (6.6) | .21 |
| New-onset AF | 21 (10.2) | 8 (8.8) | .71 |
| Treatment and prosthetic function at discharge | |||
| Type of DOAC | — | — | |
| Apixaban | 36 (39.5) | ||
| Rivaroxaban | 25 (27.5) | ||
| Edoxaban | 23 (25.3) | ||
| Dabigatran | 7 (7.7) | ||
| Adjusted DOAC dose | - | 41 (45) | |
| Aspirin | 45 (22.1) | 15 (16.9) | .35 |
| Clopidogrel | 20 (9.8) | 6 (6.7) | .51 |
| DAPT at discharge | 6 (2.9) | 4 (4.4) | .50 |
| Mean transvalvular gradient, mmHg | 8.3±4.6 | 7.5±3.7 | .28 |
| Moderate-severe or severe aortic regurgitation | 3 (1.46) | 0 | .55 |
AF, atrial fibrillation; DAPT, dual antiplatelet therapy; DOACs, direct oral anticoagulants; VKAs, vitamin K antagonists.
Values represent No. (%) or mean±standard deviation.
In-hospital events are shown in table 2. No significant differences were found between the groups in the rates of major or life-threatening bleeding (3.4% with VKAs vs 2.2% with DOACs; P=.73), minor bleeding (9.2% vs 14.2%; P=.25), or the need for blood transfusion during hospitalization (12.2% vs 12.6%; P=.91). There was a tendency for a higher rate of acute renal failure in the patients receiving VKAs at discharge (11.6% vs 6.6%; P=.20) and for a higher rate of ischemic stroke in the patients receiving DOACs at discharge (5.5% vs 2.0%; P=.12). The concomitant antiplatelet therapy at discharge is shown in table 2, and there were no significant differences between the groups in the use of aspirin, clopidogrel, or dual antiplatelet therapy. No patients received more potent P2Y12 inhibitors than clopidogrel. The duration strategy was 1 month for 3 of the 10 patients receiving dual antiplatelet therapy at discharge, 3 months for 5, and 6 months for 2. The reasons for concomitant antiplatelet and anticoagulant therapy are detailed in table 1 of the supplementary data.
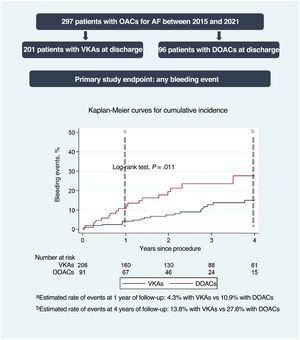
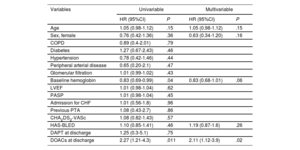
The median overall length of follow-up was 2.8 [interquartile range, 1.6-4.2] years. During follow-up, 26 patients (12.6%) receiving VKAs and 19 (20.9%) receiving DOACs experienced bleeding events. The overall incidence of bleeding events was 5.7/100 patient-years of follow-up (9.7 in the DOAC group and 4.2 in the VKA group). Patients receiving DOACs had a higher risk of bleeding events than those receiving VKAs (HR, 2.27; 95%CI, 1.21-4.26; P=.011). The cumulative incidence curves estimated with the Kaplan-Meier method are shown in figure 2. These results were concordant after adjustment for age, sex, and baseline renal function (HR, 2.30; 95%CI, 1.21-4.34; P=.01). Univariable and multivariable analyses of the primary endpoint of any bleeding event are shown in table 3.
Central illustration. Cumulative incidence of bleeding events at 4 years of follow-up by anticoagulant therapy group at discharge after TAVI (VKAs vs DOACs). AF, atrial fibrillation; DOACs, direct oral anticoagulants; OACs, oral anticoagulants; VKAs, vitamin K antagonists.
aEstimated rate of events with the Kaplan-Meier method at 1 year of follow-up: 4.3% with VKAs vs 10.9% with DOACs.
bEstimated rate of events with the Kaplan-Meier method at 4 years of follow-up: 13.8% with VKAs vs 27.6% with DOACs.
Univariable and multivariable Cox regression analysis of the primary endpoint of any type of bleeding event
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.05 (0.98-1.12) | .15 | 1.05 (0.98-1.12) | .15 |
| Sex, female | 0.76 (0.42-1.36) | .36 | 0.63 (0.34-1.20) | .16 |
| COPD | 0.89 (0.4-2.01) | .79 | ||
| Diabetes | 1.27 (0.67-2.43) | .46 | ||
| Hypertension | 0.78 (0.42-1.46) | .44 | ||
| Peripheral arterial disease | 0.65 (0.20-2.1) | .47 | ||
| Glomerular filtration | 1.01 (0.99-1.02) | .43 | ||
| Baseline hemoglobin | 0.83 (0.69-0.99) | .04 | 0.83 (0.68-1.01) | .06 |
| LVEF | 1.01 (0.98-1.04) | .62 | ||
| PASP | 1.01 (0.98-1.04) | .45 | ||
| Admission for CHF | 1.01 (0.56-1.8) | .96 | ||
| Previous PTA | 1.08 (0.43-2.7) | .86 | ||
| CHA2DS2-VASc | 1.08 (0.82-1.43) | .57 | ||
| HAS-BLED | 1.10 (0.85-1.41) | .46 | 1.19 (0.87-1.6) | .26 |
| DAPT at discharge | 1.25 (0.3-5.1) | .75 | ||
| DOACs at discharge | 2.27 (1.21-4.3) | .011 | 2.11 (1.12-3.9) | .02 |
COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; DOACs, direct oral anticoagulants; HR, hazard ratio; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; PTA, percutaneous transluminal angioplasty.
Subanalysis with bleeding types 3 and 4 of VARC-3 as endpoint revealed a statistical tendency for a higher bleeding risk in patients receiving DOACs vs VKAs (log-rank test, 0.05; VKAs vs DOACs, 1.8 and 4.7 events/100 patient-years) (figure 1 of the supplementary data).
The characteristics of the bleeding events in both groups are presented in table 4. The most frequent bleeding location was gastrointestinal in both groups (30.8% in the VKA group and 21% in the DOAC group), followed by urogenital (11.5% of bleeding events in the VKA group and 15.8% in the DOAC group). There were no significant differences in the relative distribution of bleeding severity according to VARC-2 (30.7% of bleeding events classified as major or life-threatening in the VKA group vs 31.5% in the DOAC group; P=.95) and VARC-3 (42.3% of types 3 or 4 bleeding events in the VKA group vs 47.3% in the DOAC group; P=.77).
Bleeding events after discharge in the 2 groups
| VKAs (n=206) | DOACs (n=91) | P | |
|---|---|---|---|
| VARC-2 | .77.95a | ||
| Life-threatening | 5 (19.2) | 3 (15.8) | |
| Major bleeding | 3 (11.6) | 3 (15.8) | |
| Minor bleeding | 18 (69.2) | 13 (68.4) | |
| VARC-3 | .57.77b | ||
| Type 1 | 6 (23.1) | 5 (26.3) | |
| Type 2 | 9 (34.6) | 5 (26.3) | |
| Type 3 | 8 (30.8) | 8 (42.1) | |
| Type 4 | 3 (11.4) | 1 (5.3) | |
| Bleeding location | |||
| Gastrointestinal | 8 (30.8) | 4 (21) | |
| Urogenital | 3 (11.5) | 3 (15.8) | |
| Hemorrhagic stroke | 2 (7.7) | 0 | |
| Subdural hematoma | 3 (11.5) | 2 (10.5) | |
| Retroperitoneal | 1 (3.9) | 1 (5.3) | |
| Pulmonary | 1 (3.9) | 0 | |
| Nasal/oropharyngeal | 4 (15.4) | 3 (15.8) | |
| Hemarthrosis | 1 (3.9) | 3 (15.8) | |
| Intraocular | 0 | 1 (5.3) | |
| Other | 3 (11.5) | 2 (10.5) | |
| Antiplatelet therapy at the time of bleeding | |||
| SAPT | 3 (11.5) | 2 (10.5) | |
| DAPT | 0 | 1 (5.3) | |
| None | 23 (88.5) | 16 (84.7) | |
| OAC regimen after bleeding | |||
| Unchanged | 13 (50) | 10 (52.6) | |
| Dose reduction | - | 1 (5.3) | |
| OAC withdrawn | 8 (30.8) | 8 (42.1) | |
| Switch to other OAC | 4 (15.4) | 0 | |
DAPT, dual antiplatelet therapy; OACs, oral anticoagulants; SAPT, single antiplatelet therapy; VARC, Valve Academic Research Consortium.
A total of 15 patients experienced a stroke during follow-up, 10 (4.9%) in the VKA group and 5 (5.5%) in the DOAC group. There were no significant differences in the rates of stroke between the 2 groups (HR, 1.28; 95%CI, 0.4-4.3; P=.68). The cumulative incidence curves estimated with the Kaplan-Meier method for the secondary endpoint of stroke are shown in figure 3A.
There were also no significant differences in the secondary endpoints of heart failure hospitalization (HR, 0.92; 95%CI, 0.46-1.86; P=.84) and all-cause death (HR, 1.02; 95%CI, 0.68-1.55; P=.91) (figure 3B, C).
DISCUSSIONThe main findings of our study can be summarized as follows: a) patients who underwent TAVI and required anticoagulant therapy exhibited an elevated incidence of bleeding events (> 5 events/100 patient-years of follow-up); b) patients who were discharged with DOAC therapy had a higher rate of late bleeding events than those treated with VKAs; and c) there were no significant differences between DOAC and VKA groups in the rates of stroke, heart failure, and all-cause death.
Bleeding risk has always been a relevant clinical issue in the TAVI population. First, severe aortic stenosis has been associated with a decrease in von Willebrand factor levels due to the shear stress associated with the turbulent valve flow, which promotes bleeding in some patients. In addition, both early (ie, procedure-related) and late bleeding events have a prognostic impact in TAVI patients.12,13 Late bleeding risk in TAVI patients is poorly documented. Clinical trials such as PARTNER 1 and 2 (in patients with moderate-to-high risk, as in our cohort) report bleeding rates of about 15% at 1 year of follow-up, although these studies additionally included procedure-related events.1,14 In a cohort of 372 consecutive TAVI patients, the rate of bleeding events at 1 year of follow-up was 11%, and those with significant paravalvular leak had a higher risk.15 In our cohort, the overall cumulative incidence at 4 years was 19%, indicating that this is a late complication of considerable impact.
Antithrombotic therapy in TAVI patients has been subject to debate since this treatment was introduced and is one of the main factors affecting residual bleeding risk after TAVI. For patients not requiring oral anticoagulation, the single antiplatelet therapy strategy has been extended in recent years, given its better safety profile (vs dual antiplatelet therapy) and absence of a negative impact on ischemic risk.7,16
However, the antithrombotic treatment of TAVI patients requiring anticoagulation for AF is still controversial, with few studies obtaining robust evidence on the topic. It seems clear that the addition of antiplatelet therapy to baseline anticoagulant therapy (except if strictly necessary due to recent coronary revascularization) has not shown any benefit and even markedly increases bleeding events.17 Regarding the selection of the optimal anticoagulant agent, the ENVISAGE clinical trial showed a greater bleeding risk in TAVI patients receiving edoxaban than in those receiving VKAs (9.7 and 7 events/100 person-years of follow-up).9 These data are in line with those of our series, although no significant differences were found in our cohort in the types of bleeding, while the ENVISAGE trial ultimately showed an increase in gastrointestinal bleeding with edoxaban. The ATLANTIS (Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis) study compared apixaban with the standard of care.18 In patients with AF requiring chronic oral anticoagulation (vs VKAs in the control group), no significant differences were seen regarding the safety bleeding endpoint or in the remaining clinical outcomes between these 2 treatments (apixaban vs VKAs). Notably, the length of follow-up in these 2 studies was relatively short, with outcomes at 1 and 2 years after TAVI, respectively, whereas the median follow-up in our cohort was close to 3 years. In addition, the rates of concomitant antiplatelet therapy in our cohort were low (30% vs 60% in the ENVISAGE trial). Moreover, a very high rate of patients (90%) had a previous diagnosis of AF and were anticoagulated. We believe that this could have led to better therapeutic control after the procedure in the patients receiving VKAs (most of whom were not receiving de novo VKAs), which resulted in low rates of bleeding complications in this group.
Previous observational studies have compared the 2 anticoagulation strategies. The analysis by Jocheim et al.19 revealed similar bleeding rates but higher ischemic risk in patients receiving DOACs.19 Some studies have reported data that conflict with those obtained in our cohort, indicating a higher overall risk of bleeding in patients receiving VKAs.20,21 These differences may be because AF is not the only reason for anticoagulant therapy,21 which could partly bias the results, because patients with a mechanical mitral prosthesis (treated with VKAs) generally exhibit above-average bleeding and mortality risks while patients without AF and receiving anticoagulant therapy due to pulmonary embolism typically have lower ischemic and bleeding risks. Another study22 that has reported results in the same line is limited by the fact that the patients under treatment with VKAs were the oldest segment of the cohort that underwent TAVI, and also had a higher rate of dual antiplatelet therapy at discharge. Accordingly, the main factors explaining our results are, on the one hand, the low rate of concomitant antiplatelet therapy at discharge and, on the other hand, the elevated prevalence of AF and chronic anticoagulation before the procedure.
Another relevant study is the FRAIL-AF trial,23 which, despite not including patients who underwent TAVI, did include elderly patients (mean age of 83 years, similar to our cohort). The study results indicated a higher incidence of bleeding in patients randomized to switch from VKAs to DOACs vs those who maintained their previous treatment with VKAs. The incidence of bleeding in that study was practically twice that in our sample (17.8 and 10.5 events/100 patient-years vs 9.3 and 4.4, respectively) because the patients were extremely frail.
Our study is not a randomized clinical trial but we should highlight the good comparability of the characteristics of the groups. In addition, while the main risk factors for bleeding (age, female sex, baseline hemoglobin, concomitant use of antiplatelet agents, significant paravalvular leak after TAVI) were similarly distributed between the groups, renal function was better in the DOAC group. Because renal function is a widely recognized risk factor for bleeding, we do not believe that it affected the direction of the findings. Moreover, the results were maintained after adjustment for this variable. Another important aspect is that the patients treated with VKAs in our cohort had a well-controlled international normalized ratio (INR), with a time in therapeutic range of 71%. Good control was also seen in the ENVISAGE (68.2%) and FRAIL-AF (65%-74%) trials. These results are likely not generalizable to populations with poor control of their anticoagulant therapy or to those that are just starting the therapy.
Regarding the location and magnitude of the bleeding, the tendency was maintained in a subanalysis of major bleeding (classified as type 3 or 4 according to VARC-3), which is why the study findings cannot be specifically applied to mild or minor bleeding. Nonetheless, an important finding was that there were 2 hemorrhagic stroke events in the VKA group and none in the DOAC group because this is one of the clinical events for which DOACs have previously exhibited superiority.
Several strategies could aid in the management of bleeding risk and antithrombotic therapy in our TAVI patients. First, we should not alter the VKA therapy in patients who have optimal dosage control and show no relevant clinical events. Second, we should appropriately adjust the antithrombotic therapy and discontinue antiplatelet agents whenever possible. Third, we should consider alternative strategies such as the combined use of TAVI and an atrial appendage closure device, which demonstrated noninferiority to optimal medical therapy in a composite of all-cause mortality, stroke, and major bleeding at 2 years in a recent clinical trial awaiting publication (NCT03173534).24
LimitationsOur study has several limitations. The work comprised a single-center observational study. However, the single-center setting permitted strict follow-up of the patients and access to their complete medical records, which avoided loss to follow-up and of relevant events. Because of the absence of a strict protocol governing the use of specific drugs, the sample was more likely to be comparable between groups, although there may still have been some unknown confounding and umbalanced factors, given the observational nature of the study. In Spain, DOAC therapy requires a medical necessity review, which can introduce selection bias. Nonetheless, none of the patients in our cohort discharged with a DOAC required another switch to a VKA due to medical necessity review failure. Accordingly, we believe the influence of this aspect to be minor. In addition, the data on time in therapeutic range during the clinical follow-up of patients receiving VKAs are difficult to obtain, which could introduce underdosing bias. The study did not adhere to SAGER guidelines for sex and gender. Finally, the sample size impeded direct comparisons between different DOACs or between individual DOACs and VKAs. Such analysis will require further real-world clinical practice studies.
CONCLUSIONSIn elderly patients receiving anticoagulant therapy for AF who undergo TAVI, the use of DOACs vs VKAs is associated with an increase in late bleeding events but not with significant differences in the rates of stroke or all-cause death.
- -
The optimal anticoagulant therapy of choice for patients with AF who undergo TAVI is still unknown. A recent clinical trial showed that the use of edoxaban is associated with a higher bleeding risk in these patients. In addition, in frail and elderly patients with good vitamin K antagonist control, the switch to direct oral anticoagulants is associated with higher bleeding risk than continuation with the previous anticoagulant therapy.
- -
In a real-world clinical practice cohort of patients with AF who underwent TAVI, the use of direct oral anticoagulants was associated with higher overall risk of bleeding events in 4 years of follow-up vs the use of vitamin K antagonists.
No funding was received for the preparation of this article.
ETHICAL CONSIDERATIONSThe study adhered to the recommendations of the Declaration of Helsinki. The local Health Care Ethics Committee approved the data collection and study protocol. All patients provided written informed consent for the procedure and data collection.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence has not been used in the preparation of this work.
AUTHORS’ CONTRIBUTIONSConcept, design, manuscript drafting, literature search, and statistical analysis: A. Alperi, R. Ptaszynski, and P. Avanzas. Analysis and interpretation: all authors. Critical revision: all authors. Final approval of the manuscript: all authors. A. Alperi and R. Ptaszynski share first authorship on this manuscript.
CONFLICTS OF INTERESTP. Avanzas is an associate editor of Rev Esp Cardiol; he has adhered to the editorial procedure established by the journal to guarantee the impartial management of the manuscript. The other authors have no conflicts of interest.