To the Editor:
We present the case of a 12-month-old patient, echocardiographically diagnosed with tetralogy of Fallot, with indications for surgical treatment due to cyanosis. In the hemodynamic study, subaortic interventricular communication (IVC) was found as well as aortic override, infundibular, valvular, and supravalvular pulmonary stenosis, together with L-malposition of the great arteries and the right coronary artery that crosses the pulmonary infundibulum very close to the valvular plane (Figure).
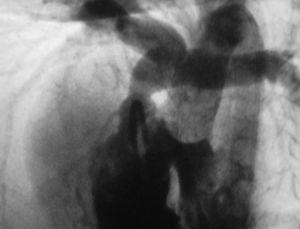
Figure. Angiography of the patient showing L-malposition of great vessels with interventricular communication (IVC) and pulmonary stenosis (infundibular, valvular, and supravalvular). The right coronary artery crosses the right ventricular outflow tract proximal to the valvular plane.
Interventricular communication was closed using a polytetrafluoroethylene (PTFE) patch via the atrial route, the pulmonary artery was sectioned and directly reimplanted onto the right ventricle, with infundibular resection and enlargement of the anterior wall of the pulmonary outflow tract using an autologous pericardial patch without prosthetic conduit (Lecompte procedure or REV1). A Lecompte maneuver was not necessary. After correction, the RV/LV pressure ratio was 0.4, without residual pulmonary gradient. There were no complications at 12-month follow-up.
The anatomically corrected malposition of great arteries is an infrequent type of conotruncal malformation where there is ventriculoarterial concordance despite presenting great vessel malposition. Thus, if there is atrioventricular concordance, the circulatory physiology is normal and surgical treatment consists in correcting the associated lesions, usually interventricular communication with infundibular pulmonary stenosis. It can also be associated with tricuspid hypoplasia/atresia with right ventricular hypoplasia, right aortic arch, juxtaposition of atrial appendages and dextrocardia.2
In this malformation, the correction of the pulmonary stenosis is determined by the position of the right coronary artery in the right ventricular infundibulum, which usually entails using an extracardiac conduit. This involves successive reinterventions in order to replace this conduit. Morita et al3 describe a transannular enlargement technique via patch plasty of the right atrioventricular groove, applicable in patients where the coronary artery is displaced away from this. In our case, given the age of the patient and the position of the coronary arteries, we chose the Lecompte procedure, connecting the pulmonary artery directly to a right ventriculotomy. This technique preserves the growth-potential of RV-pulmonary artery continuity and avoids the reinterventions due to the extracardiac conduit, making it especially attractive for use in young children. Recent studies comparing both procedures demonstrate significantly greater late morbidity with the conventional technique, which is associated with reintervention due to conduit obstruction.4,5
In the Lecompte procedure, most reinterventions due to pulmonary restenosis are associated with the use of patches with a pericardial monocusp valve which calcifies and induces obstruction.6,7 The use of a patch without a monocusp valve, as in our case, bypasses this problem, but it produces a pulmonary regurgitation that can cause late right ventricular dysfunction.
In the anatomically corrected L-malposition of great vessels with pulmonary stenosis, the Lecompte procedure offers an excellent alternative to RV-pulmonary artery conduit, since it enables earlier correction and reduces the need for reinterventions.