In cardiology anticoagulants are largely used to reduce thromboembolic complications in patients with acute coronary syndromes (ACS) or undergoing percutaneous coronary intervention (PCI), and in patients with mechanical heart valves or in those with atrial fibrillation (AF) and a CHADS2 (congestive heart failure, hypertension, age ≥75, diabetes, history of stroke or transient ischemic attack) score ≥2 or concomitant valve disease.1 Among ACS patients enrolled in clinical trials, the risk of cardiovascular death, nonfatal myocardial infarction (MI) and stroke during the first year after hospitalization remains high (approximately 10%) despite the best available antithrombotic therapies.2 Potential alternatives to heparins and to current vitamin K antagonists (VKA) include direct antagonists of coagulation factor (F) Xa ("xabans"), direct thrombin inhibitor (DTI), dabigatran etexilate, and a new VKA, tecarfarin, all in fairly advanced phases of clinical development (Figure and Table).3-10
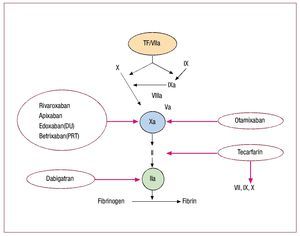
Figure 1. Scheme of coagulation. Broken arrows indicate sites of inhibition by the various compounds. FVIIa: factor VIIa; TF: tissue factor. Modified from Weitz et al3
Xabans are small, synthetic, powerful molecules (inhibitor dissociation constant [Ki] in the low nanomolar range) that reversibly inhibit the catalytic subunit of active FX, both in the soluble phase or bound within the prothrombinase complex.11 Inhibition is direct, without intermediaries such as antithrombin. Given linear pharmacokinetics and lack of food interactions, routine monitoring of the anticoagulant effect is not required.11 Many xabans are absorbed orally, with a tmax (time to maximum plasma concentration) of about 2 h and plasma half-lives (t1/2) of about 10 h. Notable exceptions are otamixaban (Sanofi-Aventis), given intravenously (IV), with a rapid onset and a t1/2 of 30 min,6 and betrixaban (Merck/Portola Pharmaceuticals), with a half-life of 19 h and almost exclusive excretion in the bile.11 Renal clearance is >50% for edoxaban tosylate (Daiichi Sankyo), about 1/3 for rivaroxaban (Xarelto®, Bayer/Johnson&Johnson) and for apixaban (Bristol-Myers Squibb/Pfizer), 10%-30% for otamixaban, and very marginal for betrixaban.6,11 Bioavailability is good for all oral xabans.11
The oral DTI dabigatran etexilate (Pradaxa®, Boehringer Ingelheim) is a prodrug that is rapidly converted to active dabigatran in the gut, plasma and liver. Dabigatran binds univalently and reversibly to both soluble and bound FIIa (ie, thrombin). It appears to be non-hepatotoxic. Its oral bioavailability is low (about 7%). Plasma levels peak 2 h after oral intake. The circulating t1/2 is about 15 h.9 Its predominantly renal excretion makes it contraindicated in patients with renal failure.9 Unlike rivaroxaban and apixaban, it is not influenced by the cytochrome P450 (CYP) system. Given the predictable pharmacokinetics, it does not require regular monitoring.9 The dose should be reduced by about 1/4 with amiodarone intake, whereas quinidine should be avoided (Pradaxa® insert). Rivaroxaban and dabigatran etexilate are commercially available and approved in Europe, Canada, and the United Kingdom for the prevention of venous thromboembolism after total hip or knee replacement. Tecarfarin (ARYx Therapeutics) is an oral VK epoxide reductase antagonist with a mean terminal half-life of 119 h. Its mechanism of action is identical to that of warfarin and monitored by the international normalized ratio (INR).10 Unlike warfarin, tecarfarin is metabolized by esterases, escaping the effects of CYP-mediated drug or food interactions and of genetic variations within the CYP system.10
New Anticoagulants in Patients With ACS or Undergoing Elective Percutaneous Coronary interventions
Rivaroxaban, apixaban, otamixaban, and dabigatran have been tested for safety, tolerability and pilot efficacy in published, randomized, double-blind, dose-finding, phase II trials (Table).4-9 Phase III studies with 3 FXa inhibitors are ongoing.
FXa Inhibitors
1. Rivaroxaban in ACS. ATLAS ACS-TIMI 46 tested 4 rivaroxaban doses (5, 10, 15, and 20 mg total daily dose given in 1 or 2 administrations) versus placebo in 3491 patients with ACS (52% ST-elevation MI, 10% non-ST-elevation MI, 17% unstable angina, 64% treated by PCI) enrolled 1-7 days after admission and followed for 6 months. Patients were stratified by the investigators' decision to use aspirin only (n=761) or aspirin plus a thienopyridine (n=2730). The incidence of Thrombolysis In Myocardial Infarction (TIMI) major and minor bleeding, or requiring medical attention, increased with increasing doses of rivaroxaban: 6.1%, 10.9%, 12.7%, and 15.3% versus 3.3% for placebo (P<.0001). Rates of death, nonfatal MI, stroke, or severe recurrent ischemia requiring revascularization (primary efficacy endpoint) were 5.6% for rivaroxaban versus 7% for placebo (hazard ration [HR] =0.79; P=.10), while the rates of the main secondary efficacy endpoint (death, nonfatal MI, or nonfatal stroke) were 3.9% versus 5.5% (HR=0.69; P=.027).4 ATLAS ACS 2-TIMI 51, an event-driven, phase III, placebo-controlled trial, is testing whether rivaroxaban (2.5 and 5 mg twice daily) for at least 6 months on top of standard care may reduce the risk of cardiovascular death, MI, or stroke in up to 16000 patients with recent ACS, at acceptable bleeding rates (NCT00809965; estimated completion June 2011).
2. Apixaban in acute coronary syndromes. APPRAISE-1 randomized 1715 patients within 7 days of an ACS (63% ST-elevation MI, 30% non-ST-elevation MI, 8% unstable angina) to 1 of 4 doses of apixaban (2.5 mg twice daily, 10 mg once daily, 10 mg twice daily, or 20 mg once daily) or to placebo for 6 months.5 Enrolled patients were clinically stable with at least 1 additional risk factor for recurrent ischemia. Nearly all received aspirin, and 76% also received clopidogrel. The 2 higher apixaban doses were discontinued for excess bleeding when combined with dual antiplatelet therapy. Bleeding rates with apixaban showed a dose-dependent increase: 5.7% for 2.5 mg bid (P=.09) and 7.9% for 10 mg od (P=.005) versus 3% for placebo. Bleeds were defined by the International Society of Thrombosis and Hemostasis (ISTH) as major (fatal, intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, intramuscular with compartment syndrome, pericardial, ≥2 g/dL fall in Hb or ≥2 units packed red blood cell transfusion) or as clinically relevant nonmajor (CRNM), ie, requiring medical or surgical intervention. Rates of cardiovascular death, nonfatal MI, severe recurrent ischemia, or ischemic stroke were 7.6% for the apixaban 2.5 mg bid dose (HR=0.73; P=.21) and 6% for the 10 mg od dose (HR=0.61; P=.07) versus 8.7% for placebo. Rates of bleeding were higher and of ischemic events lower in patients taking aspirin plus clopidogrel than in those taking aspirin alone.5 The phase III APPRAISE-2 study is testing apixaban (5 mg twice daily) versus placebo for safety and efficacy as add-on therapy in up to 11 000 high-risk patients with a recent MI (NCT00831441; estimated completion November 2011).
3. Otamixaban in percutaneous coronary interventions and acute coronary syndromes. Otamixaban has been investigated in 2 phase II trials. SEPIA-PCI randomized 947 patients undergoing nonurgent PCI to receive an IV bolus before the procedure, followed by a 3 h-infusion of either weight-adjusted unfractionated heparin (UFH) otamixaban regimens or 1 of 5 weight-adjusted. At the end of increasing otamixaban infusions, median anti-FXa activity ranged from 65 to 691 ng/mL and median aPTT (activated partial thromboplastin time) ratios from 1.1 to 2.2.6 In-hospital major or minor TIMI bleeding occurred in 2%, 1.9%, 3.8%, 3.9%, and 2.6% of patients with increasing otamixaban, and in 3.8% of controls. Ischemic events (death, MI, target-vessel revascularization) at 30 days occurred in 5.8%, 7.1%, 3.8%, 2.5%, and 5.1% of patients with increasing otamixaban, and in 5.6% of controls. Thus, the otamixaban doses 3 and 4 (corresponding to 0.12 and 0.16 mg/kg/h) were associated with lower ischemic endpoints and similar bleeding rates compared with UFH.11 In SEPIA-ACS1 TIMI 42, 3241 non-ST-elevation ACS patients undergoing early PCI received 1 of 5 IV otamixaban doses (0.08 mg/kg bolus followed by a 0.035, 0.07, 0.105, 0.14, or 0.175 mg/kg/h infusion until the end of PCI) compared to UFH (60 IU/kg IV bolus followed by a 12 IU/kg/h infusion until the end of PCI) plus eptifibatide (180 μg/kg IV bolus followed by a 1-2 μg/kg/min infusion for 18-24 h).7 The lowest otamixaban dosing was stopped early for a trend towards increased thrombotic complication rates. The incidence of all-cause death, MI, urgent revascularization, or bailout glycoprotein IIb/ IIIa inhibitor use up to 7 days was 7.2%, 4.6%, 3.8%, 3.6%, and 4.3% with increasing otamixaban (P=.34 for trend) versus 6.2% in controls. Rates of TIMI major or minor bleeding unrelated to coronary-artery bypass grafting up to 7 days were 1.6%, 1.6%, 3.1%, 3.4%, and 5.4% with increasing otamixaban (P=.0001 for trend) versus 2.7% in controls. The otamixaban bolus + 0.105-0.140 mg/kg/h infusion regimens are being considered for a phase III trial.7
Dabigatran Etexilate in Acute Coronary Syndromes
The recently completed but not yet published RE-DEEM trial (NCT00621855) compared 4 doses of dabigatran etexilate (50, 75, 110, and 150 mg bid) to placebo, in addition to dual antiplatelet treatment, in 1861 patients with ST-elevation (60%) or non-ST elevation (40%) MI at high risk for new ischemic events (29% prior MI, 31% diabetes, 12% heart failure); PCI was performed in 54%. Treatment began within 14 days (mean, 7.4) and lasted 6 months. Preliminary results indicate that ISTH major bleeding rates increased moderately with dabigatran dose (P for trend <.001), occurring in 0.8%, 0.3%, 2%, and 1.2% with increasing dabigatran, compared to 0.5% with placebo. D-dimer levels fell by 45% in all dabigatran groups out to 6 months (P<.001 vs placebo). The overall event rate of cardiovascular death, nonfatal MI and stroke was low (Oldgren J et al. Randomised dabigatran etexilate dose finding study in patients with acute coronary syndrome post index event with additional risk factors for cardiovascular complications also receiving aspirin and clopidogrel: RE-DEEM. American Heart Association scientific sessions in Orlando, FL, Nov 18, 2009).
New Anticoagulants in Patients With Atrial Fibrillation
Several phase III studies are comparing rivaroxaban, apixaban and edoxaban tosylate to warfarin or to aspirin in patients with nonvalvular AF (Table 1). Another large trial with dabigatran etexilate has been published.9 Tecarfarin and betrixaban have been investigated only in phase II studies.10
FXa Inhibitors
1. Rivaroxaban in atrial fibrillation. ROCKET AF is a double-blind, double-dummy, event-driven, phase III trial aiming to establish noninferiority of rivaroxaban (20 mg once daily) compared with warfarin (target INR 2.5) in preventing thromboembolism among 14 269 patients with nonvalvular AF and a history of stroke or at least 2 other risk factors for future stroke. The primary efficacy endpoint is the composite of stroke (ischemic and hemorrhagic) and systemic embolism. Secondary efficacy endpoints include transient ischemic attacks, all-cause death, vascular death and MI. The primary safety endpoint is the composite of major and CRNM ISTH bleeding. Patients assigned to rivaroxaban receive this drug + warfarin-placebo with sham INR values. Patients allocated to warfarin receive warfarin od + rivaroxaban-placebo. The trial started in December 2006 and is expected to last until 405 primary outcome events are observed.12
2. Apixaban in atrial fibrillation. ARISTOTLE (NCT00412984) is a double-blind, double-dummy, event driven phase III trial13 testing noninferiority of apixaban (5 mg twice daily) versus warfarin (INR 2-3) in preventing stroke and systemic embolism in 18 206 patients with nonvalvular AF and CHADS2 score ≥1 (including approximately 40% warfarin naïve). Secondary endpoints include rates of ISTH major bleeding. Planned treatment duration is 1 to 4 years, to include at least 448 primary efficacy events. As the noninferiority boundary is 1.38, apixaban will be declared noninferior if the 95% confidence interval excludes the possibility that the primary outcome rate with apixaban is >1.38 times higher than with warfarin.13
Estimated completion is in 2011. AVERROES (NCT00496769) is a double-blind, double-dummy, phase III trial comparing apixaban 5 mg twice daily versus aspirin 81 to 324 mg once daily in 5600 patients with AF and one or more risk factors for stroke who are intolerant or unsuitable for VKA.14 A recent statement announced its early closure for important reduction in stroke and systemic embolism by apixaban, at acceptable rates of major ISTH bleeding (http://www.news-medical.net/ news/20100611/Phase-3-AVERROES-clinical-trial-of-apixaban-for-atrial-fibrillation-closes-early-due-to-clear-evidence-of-efficacy. aspx. Accessed August 7, 2010). In the recent ACTIVE-A study, the combination of aspirin and clopidogrel was an effective alternative to aspirin alone for stroke prevention in AF, at the price of increased bleeding rates.14 In AVERROES, patients receiving the combination of aspirin and clopidogrel were not included.
3. Edoxaban tosylate in atrial fibrillation. A completed, phase II study randomized 1146 patients with nonvalvular AF and CHADS2 score ≥2 to receive 4 blinded edoxaban tosylate regimens (30 or 60 mg total daily doses given in 1 or 2 administrations) or open-label warfarin (INR 2-3) for 3 months. The incidence of major and CRNM ISTH bleeding in the 30 mg and 60 mg once daily treatment groups was similar to, or better than, those in the warfarin treated group.11 These doses (30 and 60 mg od) are being compared with warfarin (INR 2-3) in the double-blind, double-dummy, phase III, ENGAGE AF-TIMI 48 study for stroke prevention, enrolling approximately 16 500 patients with nonvalvular AF and Chads2 ≥2. The primary efficacy endpoint is stroke and systemic embolic events; the primary safety endpoint is major and CRNM ISTH bleeding. Expected median treatment duration is 24 months with completion estimated in the first half of 2012 (NCT 00504556).
4. Betrixaban in atrial fibrillation. The recently completed dose-finding EXPLORE-Xa phase II study randomized 508 patients with nonvalvular AF or atrial flutter and at least one risk factor for stroke to 3 blinded doses of betrixaban (40, 60, or 80 mg od) compared to open-label, dose-adjusted warfarin (INR 2-3 at maximum intervals of 4 weeks). Mean age was 74 years; 87% of patients were previously receiving a VKA; patients with severe renal impairment (but not those on dialysis) were included. After a median follow-up of 5 months, the incidence of major or CRNM bleeding was 0.8%, 3.9%, 3.2% with increasing betrixaban versus 3.2% with warfarin (P=.035 for 40 mg vs warfarin). Rates of death, stroke, MI or other systemic embolism were 0-1 in each of the 4 groups. The most common adverse events with betrixaban were diarrhea and nausea. A specific antidote for betrixaban is being developed (Ezekowitz MD et al. A phase 2, randomized, parallel group, dose-finding, multicenter, multinational study of the safety, tolerability and pilot efficacy of three blinded doses of oral factor Xa inhibitor betrixaban compared with open-label dose-adjusted warfarin in patients with non-valvular atrial fibrillation [Explore-Xa]. American College of Cardiology 59th annual scientific session in Atlanta, Ga, March 15, 2010).
Dabigatran Etexilate in Atrial Fibrillation
The phase II PETRO study randomized 502 patients with nonvalvular AF (38% permanent, 39% persistent, 23% paroxysmal) and CHADS2 score ≥1 to receive blinded doses of 50, 150, or 300 mg dabigatran etexilate twice daily —alone or combined with 81 or 325 mg aspirin— or open-label warfarin (INR 2-3) for 12 weeks.8 Major bleeding events (by ISTH-like definition) were limited to patients treated with dabigatran 300 mg bid plus aspirin, whereas thromboembolic episodes were limited to the 50 mg bid dabigatran groups.8 The subsequent phase III RE-LY study randomized 18 113 patients with AF (35% permanent, 32% persistent, 33% paroxysmal) to blinded doses of dabigatran etexilate (110 mg or 150 mg twice daily) or to unblinded adjusted-dose warfarin.9 Chads2 score was 0-1 in 32%, 2 in 35%, and 3-6 in 33% of patients (mean 2.1).9 Median follow-up was 2 years. In the groups receiving either warfarin, dabigatran 110 or dabigatran 150, the rates of the primary outcome (stroke or systemic embolism) were 1.7%/year, 1.5%/year (P<.001 for noninferiority vs warfarin) and 1.1%/year (P<.001 for superiority vs warfarin), respectively. Rates of major bleeding were 3.4%/year, 2.7%/year (P=.003) and 3.1%/year (P=.31), respectively, and of hemorrhagic stroke 0.38%/year, 0.12%/year (P<.001) and 0.10%/year (P<.001), respectively. Mortality rates were 4.1%/year, 3.8%/year (P=.13) and 3.6%/year (P=.051), respectively. MI occurred in 0.53%/year, 0.72%/year (P=.07) and 0.74%/ year (P=.048), respectively.9 Thus, compared with warfarin, dabigatran in the lower dose was as effective and generally safer; the higher dosing was more effective and as safe, with lower rates of haemorrhagic stroke but slightly increased rates of MI.
Tecarfarin in Atrial Fibrillation
An open-label, phase IIa study, lasting 6 to 12 weeks in 66 AF patients (17% permanent, 26% persistent, 58% paroxysmal) investigated the safety, tolerability and optimal dosing of tecarfarin.10 Most patients (97%) were taking warfarin at enrollment and were switched to tecarfarin. After 3 weeks of treatment, the mean interpolated time in therapeutic range was 71.4%. Only 10.9% of patients had time in therapeutic range <45%. Times in extreme INR ranges of <1.5 and >4 were 1.2% and 1.2%, respectively.10 The study suggested that tecarfarin may achieve higher rates of time in therapeutic range than warfarin. This conclusion, however, was not entirely supported by the larger, double-blind, warfarin controlled, phase II, EmbraceAC trial conducted in 612 patients (75% with AF) followed for 6-9 months. Mean interpolated time in therapeutic range was 73% for warfarin vs 74% for tecarfarin (P=.51). In the prespecified group of patients (31%) taking drugs that inhibit CYP, tecarfarin did however show significant advantage (Garcia D, Milner PG, Canafax DM, Ellis DJ. Results of the EmbraceAC trial: a head-to-head comparison of warfarin with tecarfarin, a new anticoagulant. 51st American Society of Hematology annual meeting and exposition in New Orleans, La, Dec 7, 2009).
The Future
Approval by regulatory agencies of the oral DTI dabigatran etexilate for patients with nonvalvular AF and at least 1 moderate thromboembolic risk factor is likely, given the significant net clinical benefit associated with dabigatran compared with warfarin in RE-LY.9 However, severe renal insufficiency as a contraindication to dabigatran, and careful monitoring of longterm safety, should not be overlooked. Lower dabigatran doses might be advisable among elderly or female patients or those with moderate renal insufficiency, given the greater drug exposure for a given dose in such individuals (Pradaxa® insert) and the attendant bleeding risk. Over the next year or so we should learn whether xabans, including those not prevalently cleared by the kidney, may also prove effective and safe for patients with nonvalvular AF compared with warfarin (Table).
The reversibility and relatively short half-life of dabigatran and xabans will make them attractive drugs for the perioperative management of cardiac patients with indications for these new anticoagulants, avoiding the process of "bridging." Patients with mechanical valve prostheses, so far not included in trials testing the new direct FXa and FIIa inhibitors, might benefit from tecarfarin, in virtue of its stable anticoagulant effect, as an alternative to warfarin. Results of the ATLAS and APPRAISE phase III trials —addressing the benefits and risk of rivaroxaban and apixaban on top of dual antiplatelet therapy following acute coronary syndromes— should accrue over the next 12-18 months. Preliminary results from REDEEM suggest that dabigatran etexilate can be added to dual antiplatelet therapy in ACS patients who have concomitant AF, without excessive bleeding.
Safe, effective and convenient anticoagulants will allow a greater proportion of eligible patients to actually receive appropriate treatment. In case of bleeding, however, specific antidotes for the direct FXa and FIIa inhibitors are currently lacking, nor are the laboratory tests to measure their effect well standardized. From a health economy viewpoint, the cost of these agents needs to be balanced against the savings, in terms of avoided monitoring and overall clinical benefits.
Full English text available from: www.revespcardiol.org
Correspondence: Dr. F. Andreotti,
Institute of Cardiology. Catholic University, Largo Gemelli 8. 00168 Rome. Italy
E-mail: felicita.andreotti@iol.it