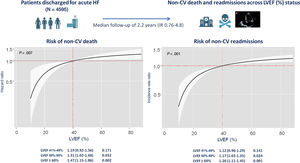
Noncardiovascular events represent a significant proportion of the morbidity and mortality burden in patients with heart failure (HF). However, the risk of these events appears to differ by left ventricular ejection fraction (LVEF) status. In this study, we sought to evaluate the risk of noncardiovascular death and recurrent noncardiovascular readmission by LVEF status following an admission for acute HF.
MethodsWe retrospectively assessed a cohort of 4595 patients discharged after acute HF in a multicenter registry. We evaluated LVEF as a continuum, stratified in 4 categories (LVEF ≤ 40%, 41%-49%, 50%-59%, and ≥ 60%). Study endpoints were the risks of noncardiovascular mortality and recurrent noncardiovascular admissions during follow-up.
ResultsAt a median follow-up of 2.2 [interquartile range, 0.76-4.8] years, we registered 646 noncardiovascular deaths and 4014 noncardiovascular readmissions. After multivariable adjustment including cardiovascular events as a competing event, LVEF status was associated with the risk of noncardiovascular mortality and recurrent noncardiovascular admissions. When compared with patients with LVEF ≤ 40%, those with LVEF 51%-59%, and especially those with LVEF ≥ 60%, were at higher risk of noncardiovascular mortality (HR, 1.31; 95%CI, 1.02-1,68; P=.032; and HR, 1.47; 95%CI, 1.15-1.86; P=.002; respectively), and at higher risk of recurrent noncardiovascular admissions (IRR, 1.17; 95%CI, 1.02-1.35; P=.024; and IRR, 1.26; 95%CI, 1.11-1.45; P=.001; respectively).
ConclusionsFollowing an admission for HF, LVEF status was directly associated with the risk of noncardiovascular morbidity and mortality. Patients with HFpEF were at higher risk of noncardiovascular death and total noncardiovascular readmissions, especially those with LVEF ≥ 60%.
Keywords
Left ventricular ejection fraction (LVEF) is the cornerstone for classification and phenotyping in heart failure (HF), as there are substantial clinical, pathophysiological and treatment differences between patients with HF with reduced EF (HFrEF), mildly reduced EF (HFmrEF), and preserved EF (HFpEF).1,2
Despite therapeutic advances, patients with HF still have a high risk of death and an enormous burden of readmissions, independently of their LVEF status.1,3,4 Cardiovascular death, mainly due to sudden cardiac death or pump failure, is the leading cause of mortality in patients with HF, and worsening HF is the leading cause of rehospitalization.1,5,6 However, the risk of cardiovascular and HF-related events seems to decrease as LVEF increases,4 and recent data from randomized clinical trials show a lower risk of cardiovascular events in patients with HFpEF.7,8
Patients with HFpEF are usually older, exhibiting a different comorbidity burden and overt pathophysiological differences to those with HFrEF.1,2,9,10 In this population, neurohormonal antagonists and other cardiovascular therapies have historically failed to show a robust clinical benefit,1 except for recent trials conducted with SGLT2 inhibitors.11,12 In HFpEF, the contribution of noncardiovascular deaths and noncardiovascular readmissions to the morbidity and mortality burden seems to be particularly relevant. 4,13–16 Noncardiovascular events are key contributors to mortality in HFpEF,8,15 and noncardiovascular readmissions seem to affect prognosis similarly to cardiovascular events.17,18 but few studies have focused on their impact.
In the present study, we sought to assess the noncardiovascular morbidity and mortality burden in an unselected population with HF and to evaluate whether modification of this risk differs along the continuum and categories of LVEF.
METHODSStudy group and protocolWe conducted a retrospective analysis of a multicenter prospective registry of 4812 consecutive patients admitted for acute HF to 3 academic hospitals in the Valencian Community (Spain) from January 2008 to October 2019. Two of the hospitals are tertiary centers with 582 and 574 beds, respectively, while the third is a 325-bed community hospital. At the index hospitalization, 217 patients died, leaving a final sample size of 4595 patients discharged alive. A comprehensive dataset of demographics, medical history, standard laboratory and echocardiographic parameters, and treatments at discharge was routinely recorded using pre-established registry questionnaires during the index hospitalization. Patients with either new-onset or worsening HF were enrolled in the registry. Acute HF was defined according to the European Society of Cardiology clinical practice guidelines. Treatment strategies were individualized following established guidelines operating when the patients were included in the registry.
EchocardiographyA 2-dimensional transthoracic echocardiogram was performed in all patients during the index hospitalization (96±24hours after admission) by experienced sonographers using the left lateral decubitus position. Commercially available systems were used throughout the study. Patients were admitted to the hospitalization ward and were clinically stable at the time of the examination. All images were recorded with the second harmonic at the time of end-expiration. LVEF was assessed by the biplane Simpson method.
Follow-up, endpoints, and ethical concernsThe incidence of both noncardiovascular mortality and recurrent noncardiovascular admissions were selected as the study endpoints. Cardiovascular death was considered secondary to worsening HF, acute myocardial infarction, stroke or transient ischemic attack, cardiac arrhythmias, peripheral artery disease, sudden cardiac death, or unknown cause of death.19 The cause of death was considered as noncardiovascular if a specific noncardiovascular cause was identified. For the readmission endpoints, only unplanned readmissions were registered. All readmissions during follow-up were classified as cardiovascular-related (including worsening HF, acute myocardial infarction, unstable angina, stroke or transient ischemic attack, cardiac arrhythmias, or peripheral artery disease). Otherwise, a noncardiovascular etiology was considered, and included cancer, infectious, gastrointestinal, renal, pulmonary, endocrine, urologic/gynecologic or rheumatologic causes. Noncardiovascular admissions were classified as infections, cancer-related, renal, and others following the primary diagnosis of the clinical report. Readmissions due to acute renal failure in the setting of worsening HF status were classified as HF-related. Those due to other causes were considered as noncardiovascular.
Follow-up and endpoint ascertainment was performed by reviewing electronic medical records from the public health care system. Endpoint adjudication was performed by paired investigators who were blinded to LVEF status.
The study conformed to the principles outlined in the 1975 Declaration of Helsinki and was approved by the local institutional ethics committee. All patients gave informed consent.
Statistical analysisContinuous variables are expressed as mean±standard deviation (SD) or median [interquartile range (IQR)], when appropriate. Discrete variables are summarized as percentages. Baseline characteristics were compared among categories with the Pearson chi-square and ANOVA tests for categorical and continuous variables, respectively. We evaluated LVEF as a continuum, stratified into 4 categories (LVEF ≤ 40%, 41%-49%, 50%-59%, and ≥ 60%). The association between LVEF status and noncardiovascular death was evaluated using a Cox regression analysis, and the results are expressed as hazard ratios (HR) with 95% confidence intervals (95%CI). Cox regression estimates were adjusted for cardiovascular death as a competing event. For the readmission endpoint, a descriptive analysis of recurrent events was performed by counting the number of hospitalizations during follow-up. Crude incidence rates (expressed as the number of readmissions per 100 person-years) were calculated for each readmission endpoint. We used bivariate negative binomial regression models that simultaneously modeled the number of noncardiovascular readmissions (as counts) and all-cause mortality (as a terminal event). Regression estimates for both outcomes were mutually adjusted by means of shared frailty (accounting for the positive correlation between the 2 outcomes).20 Risk estimates are expressed as incidence rate ratios (IRR).
The variables included in the final multivariable models were age, sex, first HF admission, prior New York Heart Association (NYHA) class III/IV, systolic blood pressure at admission, heart rate at admission, Charlson comorbidity index, plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP), blood urea nitrogen, estimated glomerular filtration rate, antigen carbohydrate 125, hemoglobin, loop diuretic dose at discharge, renin-angiotensin-aldosterone system inhibitors at discharge, beta-blockers at discharge, and mineralocorticoid receptor antagonists at discharge, accounting for cardiovascular mortality or cardiovascular readmissions as a competing event. For both multivariable models, candidate covariates were chosen based on previous medical knowledge; then, a backward stepwise selection was performed. During this selection process, the linearity assumption for all continuous variables was simultaneously tested, and the variable transformed, if appropriate, with fractional polynomials. All variables listed in table 1 were evaluated as potential covariates in the multivariable models, independently of their P value. Multiple imputations were performed for those covariates with missing values. In all cases, the rates were<5%.
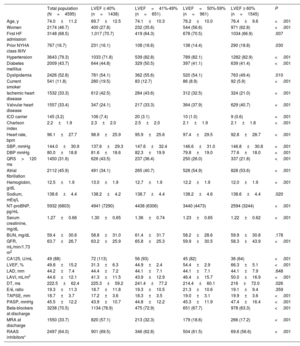
Baseline characteristics of patients across left ventricular ejection fraction categories
| Total population (N=4595) | LVEF ≤ 40% (n=1438) | LVEF=41%-49% (n=651) | LVEF=50%-59% (n=961) | LVEF ≥ 60% (n=1545) | P | |
|---|---|---|---|---|---|---|
| Age, y | 74.0±11.2 | 69.7±12.5 | 74.1±10.3 | 76.2±10.0 | 76.4±9.6 | <.001 |
| Women | 2174 (46.7) | 400 (27.8) | 232 (35.6) | 544 (56.6) | 971 (62.8) | <.001 |
| First HF admission | 3148 (68.5) | 1,017 (70.7) | 419 (64.3) | 678 (70.5) | 1034 (66.9) | .007 |
| Prior NYHA class III/IV | 767 (16.7) | 231 (16.1) | 108 (16.6) | 138 (14.4) | 290 (18.8) | .030 |
| Hypertension | 3643 (79.3) | 1033 (71.8) | 539 (82.8) | 789 (82.1) | 1282 (82.9) | <.001 |
| Diabetes mellitus | 2009 (43.7) | 644 (44.8) | 329 (50.5) | 397 (41.1) | 639 (41.4) | <.001 |
| Dyslipidemia | 2426 (52.8) | 781 (54.1) | 362 (55.6) | 520 (54.1) | 763 (49.4) | .010 |
| Current smoker | 541 (11.8) | 280 (19.5) | 83 (12.7) | 86 (8.9) | 92 (5.9) | <.001 |
| Ischemic heart disease | 1532 (33.3) | 612 (42.5) | 284 (43.6) | 312 (32.5) | 324 (21.0) | <.001 |
| Valvular heart disease | 1557 (33.4) | 347 (24.1) | 217 (33.3) | 364 (37.9) | 629 (40.7) | <.001 |
| ICD carrier | 145 (3.2) | 106 (7.4) | 20 (3.1) | 10 (1.0) | 9 (0.6) | <.001 |
| Charlson index | 2.2±1.9 | 2.3±2.0 | 2.5±2.0 | 2.1±1.9 | 2.1±1.8 | <.001 |
| Heart rate, bpm | 96.1±27.7 | 98.9±25.9 | 95.9±25.6 | 97.4±29.5 | 92.8±28.7 | <.001 |
| SBP, mmHg | 144.0±30.9 | 137.6±29.3 | 147.6±32.4 | 146.6±31.0 | 146.8±30.8 | <.001 |
| DBP mmHg | 80.0±18.8 | 81.6±18.6 | 82.3±19.9 | 79.8±19.0 | 77.6±18.0 | <.001 |
| QRS>120 ms | 1450 (31.6) | 626 (43.5) | 237 (36.4) | 250 (26.0) | 337 (21.8) | <.001 |
| Atrial fibrillation | 2112 (45.9) | 491 (34.1) | 265 (40.7) | 528 (54.9) | 828 (53.6) | <.001 |
| Hemoglobin, g/dL | 12.5±1.9 | 13.0±1.9 | 12.7±1.9 | 12.2±1.9 | 12.0±1.9 | <.001 |
| Sodium, mEq/L | 138.6±4.4 | 138.2±4.2 | 138.7±4.4 | 138.2±4.6 | 138.6±4.4 | .020 |
| NT-proBNP, pg/mL | 5932 (6803) | 4941 (7290) | 4438 (6306) | 3440 (4473) | 2594 (3244) | <.001 |
| Serum creatinine, mg/dL | 1.27±0.66 | 1.30±0.65 | 1.36±0.74 | 1.23±0.65 | 1.22±0.62 | <.001 |
| BUN, mg/dL | 59.4±30.6 | 58.8±31.0 | 61.4±31.7 | 58.2±28.6 | 59.9±30.8 | .178 |
| GFR, mL/min/1.73 m2 | 63.7±26.7 | 63.2±25.9 | 65.8±25.3 | 59.9±30.5 | 58.3±43.9 | <.001 |
| CA125, U/mL | 49 (88) | 72 (113) | 56 (93) | 45 (82) | 36 (64) | <.001 |
| LVEF, % | 49.8±15.2 | 31.3±6.3 | 44.9±2.4 | 54.4±2.9 | 66.3±5.1 | <.001 |
| LAD, mm | 44.2±7.4 | 44.4±7.2 | 44.1±7.1 | 44.1±7.1 | 44.1±7.9 | .648 |
| LAVI, mL/m2 | 44.6±12.1 | 41.3±11.5 | 43.9±12.9 | 46.4±15.7 | 50.0±16.9 | <.001 |
| DT, ms | 222.5±62.4 | 225.3±59.2 | 241.4±77.2 | 214.4±60.1 | 216±72.0 | .026 |
| E/é, ratio | 19.3±11.3 | 18.7±11.8 | 19.3±10.5 | 21.3±10.6 | 19.1±9.4 | .359 |
| TAPSE, mm | 18.7±3.7 | 17.2±3.6 | 18.3±3.5 | 19.0±3.1 | 19.9±3.6 | <.001 |
| PASP, mmHg | 45.5±12.2 | 43.9±10.7 | 44.8±12.2 | 45.3±11.9 | 47.4±16.4 | <.001 |
| Beta-blockers at discharge | 3238 (70.5) | 1134 (78.9) | 475 (72.9) | 651 (67.7) | 978 (63.3) | <.001 |
| MRA at discharge | 1550 (33.7) | 820 (57.1) | 213 (32.3) | 179 (18.6) | 266 (17.2) | <.001 |
| RAAS inhibitors* | 2497 (64.0) | 901 (69.5) | 346 (62.8) | 504 (61.5) | 69.6 (56.6) | <.001 |
BUN, blood urea nitrogen; CA125, carbohydrate antigen 125; DBP, diastolic blood pressure; DT, deceleration time; GFR, glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; LAD, left atrial diameter; LVAI, left atrium volume index; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NT-proBNP, amino-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary arterial systolic pressure; RAAS, renin-angiotensin-aldosterone inhibitors; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion.
The data given are expressed as No. (%) or mean±standard deviation.
A 2-sided P value of<.05 was considered statistically significant for all analyses. All survival analyses were performed using STATA 15.1 (StataCorp. 2015. Stata Statistical Software: Release 14.1. United States).
RESULTSThe mean±SD age of the cohort was 74.0±11.2 years, 2147 (47%) were women, and 1447 (31%) were previously admitted for HF. The number of patients with LVEF ≤ 40%, 41%-49%, 50%-59%, and ≥ 60% was 1438 (31.3%), 651 (14.2%), 961 (20.9%), and 1545 (33.6%), respectively. Baseline characteristics categorized by LVEF in the overall cohort are shown in table 1. As LVEF increased, patients were older, with a higher proportion of women, and a history of hypertension, atrial fibrillation, or valvular heart disease. Patients with HFpEF showed worse renal function or worse baseline NYHA class before admission and higher rates of prior HF hospitalizations. Patients with reduced LVEF showed a higher proportion of diabetes, ischemic heart disease, and a higher Charlson comorbidity index. Values of NT-proBNP and antigen carbohydrate 125 were lower as LVEF increased. Treatments with neurohormonal antagonists were higher as LVEF decreased, with the highest use in patients with HFrEF (table 1).
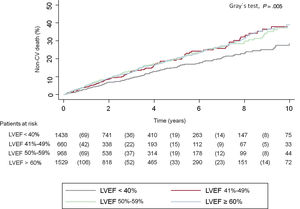
Noncardiovascular mortality risk by LVEF statusAt a median follow-up of 2.2 [0.76-4.80] years, 2257 patients had died (49%). Of them, 1611 (35%) died from cardiovascular causes and 646 (14.2%) died from noncardiovascular causes, indicating that 29% of the deaths in the overall cohort were related to noncardiovascular causes. Cumulative incidence plots showed that patients with HFrEF had the lowest incidence of noncardiovascular mortality (Gray test; P=.005), without overt differences in those patients with LVEF>40% (figure 1).
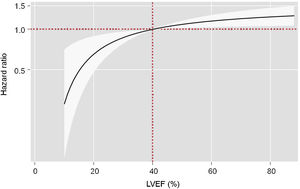
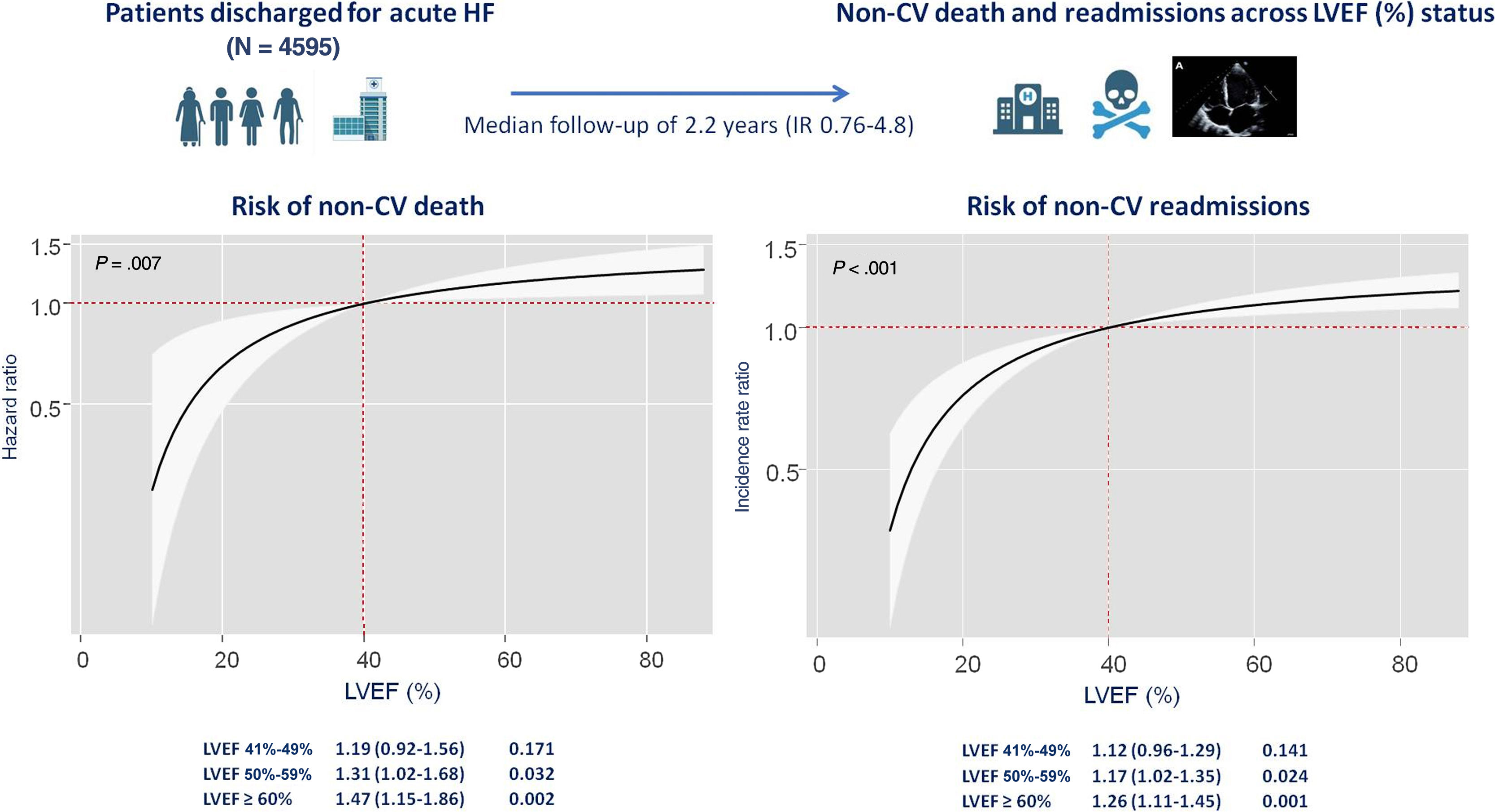
On multivariable analysis accounting for the risk of cardiovascular death as a competing event, LVEF status as a continuous variable was directly associated with the risk of noncardiovascular mortality (P=.007) (figure 2). Compared with patients with HFrEF, those with LVEF 51%-59%, and especially those with LVEF ≥ 60%, had a significantly higher risk of noncardiovascular mortality (HR, 1.31; 95%CI, 1.02-1.68; P=.032; and HR, 1.47; 95%CI, 1.15-1.86; P=.002; respectively). Patients with HFmrEF did not show a higher risk of noncardiovascular events (HR, 1.19; 95%CI, 0.92-1.56; P=.171) (table 2).
Risk estimates for the risk of noncardiovascular mortality and noncardiovascular readmissions across left ventricular ejection fraction categories in the multivariable models
| Noncardiovascular mortality | HR (95%CI) | P |
|---|---|---|
| HFrEF (reference) | ||
| LVEF=41%-49% | 1.19 (0.92-1.56) | .171 |
| LVEF=50%-59% | 1.31 (1.02-1.68) | .032 |
| LVEF ≥ 60% | 1.47 (1.15-1.86) | .002 |
| Noncardiovascular readmissions | IRR (95%CI) | P |
|---|---|---|
| HFrEF (reference) | ||
| LVEF=41%-49% | 1.12 (0.96-1.29) | .141 |
| LVEF=50%-59% | 1.17 (1.02-1.35) | .024 |
| LVEF ≥ 60% | 1.26 (1.11-1.45) | .001 |
The models were adjusted for age, sex, first HF admission, prior NYHA class III/IV, systolic blood pressure at admission, heart rate at admission, Charlson comorbidity index, plasma N-terminal pro-B-type natriuretic peptide, blood urea nitrogen, estimated glomerular filtration rate, antigen carbohydrate 125, hemoglobin, loop diuretic dose at discharge, renin-angiotensin-aldosterone system inhibitors at discharge, beta-blockers at discharge, mineralocorticoid receptor antagonists at discharge, and the risk of CV death or CV readmissions as competing events.
HR, hazard ratio; HFrEF, heart failure with reduced ejection fraction; IRR, incidence rate ratios; LVEF, left ventricular ejection fraction.
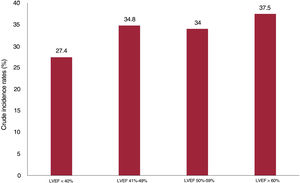
During the follow-up, 9281 all-cause readmissions were registered in 3145 patients (68.4%). Of them, 4014 rehospitalizations in 1902 patients (41.4%) were due to noncardiovascular causes. A substantial number of patients experienced recurrent noncardiovascular events during the follow-up. There were 435, 225, 103, 265, and 722 patients with 2, 3, 4, 5, and>5 rehospitalizations, respectively. The rates of noncardiovascular readmission (per 100 person-years) significantly increased from lower to higher LVEF categories (figure 3). The most frequent causes of noncardiovascular readmissions, were other causes (2134 hospitalizations), followed by infections (975 hospitalizations), cancer-related causes (578 hospitalizations), and renal causes (327 hospitalizations).
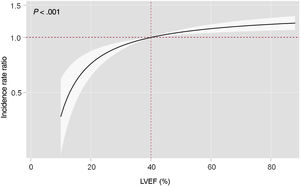
Multivariable adjustment including cardiovascular readmissions as a competing event showed that LVEF status as a continuous variable was directly associated with the risk of recurrent noncardiovascular admissions (P<.001). There was a significant stepwise increase in the risk of recurrent noncardiovascular hospitalizations in patients with higher LVEF (figure 4). Similar findings were inferred on analysis of LVEF categories. Compared with HFrEF, LVEF 51%-59% status, and especially LVEF ≥ 60%, were independently associated with a higher risk of recurrent noncardiovascular admissions (IRR, 1.17; 95%CI, 1.02-1.35; P=.024; and IRR, 1.26; 95%CI, 1.11-1.45; P=.001; respectively). This association was not observed for the HFmrEF category (IRR, 1.12; 95%CI, 0.96-1.29; P=.141) (table 2).
Detailed multivariable models including all the covariates and their risk estimates for noncardiovascular mortality and recurrent noncardiovascular admissions are shown in tables 1 and 2 of the supplementary data.
DISCUSSIONIn the current study, we evaluated the long-term burden of noncardiovascular events in a multicenter registry of patients following admission for acute HF. We confirmed the high noncardiovascular morbimortality burden of this population in daily clinical practice. More interestingly, we found that the risk of noncardiovascular events was associated with LVEF status (figure 5). This risk was substantially higher in HFpEF, especially in those patients with LVEF ≥ 60%.
Central illustration. Study protocol summary and risk estimates (HRs and IRRs) for the risks of noncardiovascular death and recurrent admissions, respectively, across the continuum of left ventricular ejection fraction in the multivariable models, and stratified by subgroups with heart failure with reduced ejection fraction as reference. CV, cardiovascular; HF, heart failure; HR, hazard ratio; IR, interquartile range; IRR, incidence rate ratios; LVEF, left ventricular ejection fraction.
Patients with HF have a substantial burden of events regardless of LVEF status.1,3–6 This is especially true following admission for acute HF, a turning point in the natural history of the disease.1,3 Cardiovascular events, such as cardiovascular death or worsening HF, are the main causes of death and readmission in HF, respectively, and are considered to be the traditional endpoints in HF.21 However, as recently observed in clinical trials and observational studies, the burden of noncardiovascular-related events is also substantial and is progressively rising.8,15,22 Despite contemporary real-world studies showing advanced age and growing comorbidity in HF, the importance and repercussion of noncardiovascular events in daily clinical practice have not been adequately evaluated. These events have historically been neglected, as they have not been considered to be directly related to the disease, are difficult to prevent and manage, and do not seem to be affected by cardiovascular therapies.14–18
Observational studies and data from randomized clinical trials have shown that noncardiovascular events are common in patients with HFpEF.8,10,14,16,18 HFpEF is a challenging syndrome, as patients are older and their comorbidity burden differs from that of patients with HFrEF.1,6,10 A recent observational study from Italy reported that noncardiovascular mortality accounted for 62% of deaths in patients with HFpEF but for only 35% of those in patients with HFrEF.23 In a previous study, patients with HFpEF showed a lower risk of HF-related death.4 Data from randomized clinical trials have reinforced the magnitude of noncardiovascular morbidity and mortality in patients with HFpEF. In a pooled analysis of the DAPA-HF and DELIVER trials, including HF patients with the entire spectrum of LVEF, 53% of the deaths were attributed to cardiovascular causes, but the proportion of deaths attributed to cardiac conditions was inversely correlated with LVEF, representing less than 40% of deaths in patients with LVEF ≥ 60%.8 The data are more scarce on readmission risk. In a recent analysis of the TOPCAT-Americas cohort, half of the readmissions during the trial were due to a noncardiovascular cause, and up to 44% of total readmissions in patients included in the I-PRESERVE trial were due to noncardiovascular causes.14,18 In agreement with these findings, a large observational study including 40 239 patients with chronic HF reported that those with HFpEF had a significantly increased risk of noncardiovascular readmissions compared with patients with HFrEF.13 Our results are concordant with previous data, showing a higher event rate and a higher risk of noncardiovascular morbidity and mortality in patients with HFpEF than in those with reduced LVEF in daily clinical practice.
The importance of these noncardiovascular events in HF should not be neglected. Both the analyses from the CHARM and TOPCAT trials reported that noncardiovascular hospitalizations were associated with a subsequent mortality risk comparable to that related to a cardiovascular event.17,18
Higher risk of noncardiovascular events in patients with supranormal LVEFThere is substantial evidence of differences among established HF phenotypes, but few studies have focused on potential differences in noncardiovascular events across the continuum of LVEF. Desai et al.8 recently showed that noncardiovascular death rates were inversely related to LVEF status evaluated as a continuum in a pooled analysis of the DAPA-HF and DELIVER cohorts. Our data expand these results to “real-world” clinical practice by showing a significant increase not only in noncardiovascular mortality but also a higher risk of noncardiovascular rehospitalization as LVEF increased.
Patients with HF in the upper range of LVEF show some distinct features compared with other patients with HF and are considered to have “supranormal” LVEF. The cutoff of supranormal LVEF is fairly arbitrary. There is currently no standard definition, but it is considered to be above 60%-65%.24–26 In our study, these patients were predominantly women and were older (median age, 76 years) and had low values of natriuretic peptides and a high prevalence of hypertension and atrial fibrillation; only 21% of the patients had ischemic heart disease. These features are concordant with data from clinical trials.7,27 Rosch et al.28 recently described significant morphologic and pathophysiological differences in patients with HFpEF and LVEF 50%-60% and those with LVEF>60%. Patients with LVEF 50%-60% shared important features with patients with either HFmrEF or HFrEF, showing reduced contractility, impaired ventriculoatrial coupling, and higher extracellular volume fraction. In contrast, patients with LVEF>60% had a hypercontractile state with excessive LV overload and reduced preload reserve.28
These distinct clinical and pathophysiological features of patients with HF with supranormal EF may have important implications. For instance, substudies of randomized clinical trials evaluating the effect of renin-angiotensin-aldosterone system inhibitors in HFpEF have shown a potential beneficial effect of treatments at the lower end of LVEF (with even positive results in patients within the LVEF 50%-60% category, especially in women).29–31 Conversely, these analyses have failed to find positive clinical results in patients with supranormal LVEF.27 In a subgroup analysis of the PARAGON-HF trial, sacubitril-valsartan reduced the primary endpoint of the study in patients with LVEF<57% but not in those in the highest end of LVEF, and the beneficial effect of empagliflozin in the EMPEROR clinical trial program was attenuated in patients with LVEF>65%.7,15 The authors of a recent analysis with dapagliflozin across the range of LVEF in a combined analysis of the DAPA-HF and DELIVER trials reported no significant attenuation of the benefit of SGTL2 inhibitors in patients in the upper extreme of LVEF. However, crude incidence rates of cardiovascular events were low and the confidence interval was broader when LVEF was>60%-65%.32 The higher proportional contribution of noncardiovascular events to the morbidity and mortality burden in patients in the upper range of LVEF may be partly responsible for the limited benefit of cardiovascular therapies. As these events are hardly affected by cardiovascular drugs, it is difficult for any cardiovascular treatment to show meaningful benefits in patients with a high burden of noncardiovascular events.
Our observational study does not elucidate the underlying mechanisms explaining the higher risk of noncardiovascular events in HF patients with LVEF in the upper range. As stated previously, it can be argued that the higher proportion of noncardiovascular morbidity and mortality in an older and comorbid population may relatively minimize the incidence of cardiovascular events. In a large nationwide registry in Australia, including nearly half a million participants referred for echocardiography, the risk of cardiovascular death started to decline at high LVEF levels (> 60-65%).25 However, a supranormal LVEF may also reflect a hyperdynamic state or a maldaptative cardiac response to some systemic pathophysiological phenomena linked to noncardiovascular events, such as anemia, tissue hypoxia or systemic inflammation, as patients with small and stiff hearts need to increase LVEF to maintain adequate cardiac output.33 This hypothesis is supported by recent findings from the HOMAGE trial, in which patients in the upper range of LVEF had higher levels of circulating cytokines and proinflammatory proteins.34 Other comorbid conditions, such as infections and cancer, have also been associated with LVEF in the upper range.35
In HFpEF and LVEF in the upper range, in which noncardiovascular events play such an important role, our data reinforce the idea of developing multidisciplinary management programs beyond cardiac-specific therapies to reduce this burden of noncardiovascular hospitalizations and death.
LimitationsFirst, this is an observational study that may be affected by hidden bias and residual confounders. Second, the attribution of causes of events in observational studies remains challenging and may show inaccuracies; in our study, there was no external event adjudication commitee. Indeed, we simply registered some specific causes of noncardiovascular events, and in most cases, the specific cause of readmission was not registered. Third, the LVEF cutoff point of 60% may seem arbitrary, as other studies have used other cutoffs (such as 65%). However, this classification has been used in important recent studies, such as the pooled analysis of the DAPA-HF and DELIVER cohorts, and has a pathophysiological basis.8,23 Fourth, HF etiology was not verified in all patients, and the specific contribution of specific emerging etiologies such as amyloidosis could not be examined. Fifth, data on body mass index are missing, precluding us from evaluating the potential implications of obesity on the incidence of noncardiovascular events and the supranormal LVEF phenotype. Finally, pathophysiological mechanisms underlying our findings are beyond the scope of our study, and future works should confirm these findings and explore the underlying causes.
CONCLUSIONSAfter admission for acute HF, LVEF status was associated with the risk of noncardiovascular morbidity and mortality. Patients with HFpEF, especially those with LVEF ≥ 60%, had a higher risk of noncardiovascular death and noncardiovascular total readmissions.
- -
Noncardiovascular events represent a significant and increasing proportion of the morbidity and mortality burden in patients with HF.
- -
However, the risk of noncardiovascular events appears to differ across LVEF status.
- -
LVEF status was directly associated with the risk of noncardiovascular morbidity and mortality.
- -
Patients with HFpEF had a higher risk of noncardiovascular death and noncardiovascular total readmissions, especially those with LVEF ≥ 60%.
- -
HF management programs should take into account the high risk of noncardiovascular events, especially in patients in the upper range of LVEF.
Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares, Spain (grant numbers 16/11/00420 and 16/11/00403).
AUTHORS’ CONTRIBUTIONSE. Santas, P. Llácer, and J. Núñez: concept, design, data collection, statistical analysis, manuscript elaboration, manuscript review. P. Palau, R. de la Espriella, G. Miñana: concept, design, data collection, manuscript review. Ò. Miró, F. J. Chorro, A. Bayés-Genís, J. Sanchis: concept, design, manuscript review. M. Lorenzo, G. Núñez-Marín: data collection, manuscript review.
CONFLICTS OF INTERESTJ. Sanchis is editor-in-chief of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed; he also declares speaker fees from Astra Zeneca, Abbott, and Edwards Lifesciences (modest). J. Núñez received board speaker fees and travel expenses from Novartis, Roche Diagnostics, Abbott, Rovi, Vifor Pharma, Daiichi Sankyo, Boehringer Ingelheim, and Astra Zeneca (modest). A. Bayés-Genís received board membership fees and travel expenses from Novartis, Roche Diagnostics, Vifor Pharma, and Critical Diagnostics (modest). The remaining authors have no disclosures to report.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.05.005