Cardiac resynchronization therapy (CRT) is beneficial for selected heart failure (HF) patients, although nonresponse to therapy is still prevalent. We investigated a set of novel biomarkers associated with various pathophysiological pathways of HF. Our purpose was to assess their ability to predict clinical outcomes after CRT.
MethodsWe studied 136 chronic HF patients undergoing CRT. We measured the plasma levels of fractalkine, pentraxin-3, hepatocyte growth factor (HGF), carbohydrate antigen-125, and matrix metalloproteinase-9 before and 6 months after CRT. The primary endpoint of the study was 5-year all-cause mortality, and we considered the absence of 6-month reverse remodelling (defined as at least a 15% decrease in end-systolic volume) as a secondary endpoint.
ResultsFifty-eight patients died during the 5-year follow-up period and 66 patients were categorized as nonresponders. In multivariable models, only an increased HGF was an independent predictor of both mortality (HR, 1.35; 95%CI, 1.11-1.64; P=.003; per 1 standard deviation increase) and the absence of reverse remodelling (OR, 1.83; 95%CI, 1.10-3.04; P=.01; per 1 standard deviation increase). Applying HGF to the basic multivariable model of both mortality (net reclassification improvement=0.69; 95%CI, 0.39-0.99; P<.0001; integrated discrimination improvement=0.06; 95%CI, 0.02-0.11) and reverse remodelling (net reclassification improvement=0.39; 95%CI, 0.07-0.71; P=.01; integrated discrimination improvement=0.03; 95%CI, 0.00-0.06) resulted in a statistically significant reclassification and discrimination improvement.
ConclusionsOf the investigated biomarkers, only HGF predicted clinical outcomes following CRT independently of other parameters. Reclassification analyses showed that HGF measurements could be useful in refining patient selection.
Keywords
Cardiac resynchronization therapy (CRT) is an effective treatment for patients with heart failure (HF), severely reduced ejection fraction, and wide QRS complex.1 Cardiac resynchronization therapy increases ejection fraction and reduces left ventricular volumes, resulting in reverse remodelling. The patients’ functional capacity improves, while morbidity and mortality decrease. Despite optimal patient selection, approximately 20% to 40% of patients do not respond to CRT.2 The need for novel biomarkers that can identify such patients is therefore crucial. Numerous biomarkers related to inflammation, tissue remodelling, or neurohormonal activation have been proposed to have a potential impact in clinical practice.3–8
In this study, we aimed to investigate a set of novel HF biomarkers associated with various pathways of HF pathophysiology. These biomarkers have been shown to correlate with HF outcomes in previous studies, but to date no data are available about their role in predicting outcomes after CRT. Fractalkine has been regarded as a complex inflammatory mediator, acting as an adhesion molecule in its membrane-bound form and as a chemoattractant in its soluble form.7 Pentraxin-3 functions as a mediator of innate immunity and is produced mostly at the site of inflammation.6 Hepatocyte growth factor (HGF) is a protective agent in heart diseases, exerting angiogenic, antifibrotic and antiapoptotic effects by inducing classic tyrosine kinase pathways.3 Carbohydrate antigen-125 is a well-known biomarker of ovarian cancer, but its role in acute and chronic HF has also been proposed recently, as it might reflect the contractile status of the heart and fluid overload.4 Matrix metalloproteinase-9 is responsible for proteolytic degradation of the myocardial extracellular matrix; hence, it plays a key role in myocardial remodelling.5,8
Our purpose was to assess the ability of these biomarkers in the prediction of clinical outcomes after CRT. We hypothesized that their baseline levels would predict the 6-month reverse remodelling and 5-year all-cause mortality in HF patients undergoing CRT. We also hypothesized that their prediction could be independent of (and therefore offer additional value to) N-terminal pro-B-type natriuretic peptide (NT-proBNP), which is regarded as the gold standard blood biomarker in HF.9 We proposed to prove our hypotheses with reclassification models.
METHODSWe enrolled 141 consecutive chronic HF patients in our prospective, single-center observational study. We aimed to evaluate the prognostic impact of novel laboratory biomarkers in chronic HF patients with CRT in a previously described cohort.10 The present analysis focuses on the impact of novel HF biomarkers in CRT.
The patients were on optimal medical therapy and underwent CRT implantation between September 2009 and December 2010 according to current guidelines11 in the Heart and Vascular Center of Semmelweis University, Budapest. Inclusion criteria included stable chronic HF, optimal medical treatment, New York Heart Association (NYHA) functional class II-IVa, wide QRS (> 120 msec) in the electrocardiogram irrespective of morphology and severely reduced left ventricular ejection fraction (under 35%). We regarded severe systemic inflammatory and hematologic diseases and malignancies as exclusion criteria, and 5 patients were excluded on this basis.
The primary endpoint of the study was 5-year all-cause mortality, and we considered 6-month reverse remodelling, defined as at least a 15% decrease in left ventricular end-systolic volume, as a secondary endpoint. All patients provided their written informed consent before enrolment. The investigation conformed with the principles outlined in the Declaration of Helsinki. The study protocol was approved by the local Ethics Committee.
The patients were followed up for 5 years, with visits at 6 months, 2 years and 5 years after CRT implantation. Clinical examinations, laboratory measurements, electrocardiogram and echocardiography at baseline were performed in 136 patients, and their data were included in the final analysis. At each follow-up visit, we evaluated the patients’ functional status by assessing the NYHA functional class, their medical therapy, and relevant adverse medical events. Repeated laboratory measurements, echocardiography and electrocardiogram were taken at 6 months.
We obtained venous blood samples, then processed the serum and ethylenediaminetetra-acetic acid plasma aliquots within 2hours. Samples were stored at –80°C for later biomarker measurements. NT-proBNP levels were measured with electrochemiluminescence technology using a Cobas e 411 analyzer (Mannheim, Germany) with Roche Elecsys NT-proBNP II kits (Cat. No.: 04842464190, Mannheim, Germany). Commercially available enzyme-linked immunosorbent assays were used for measuring fractalkine (Human CX3CL1/Fractalkine Quantikine ELISA Kit, R&D Systems, Minneapolis, United States, Cat. No.: RD-DCX310), pentraxin-3 (Human Pentraxin3 / TSG-14 ELISA System, CosmoBio, Tokyo, Japan, Cat. No.: PPX-PP-PD03-E0), HGF (Human HGF Quantikine ELISA Kit, R&D Systems, Minneapolis, United States, Cat. No.: RD-DHG00) and matrix metalloproteinase-9 (Human MMP-9, Quantikine ELISA Kit, R&D Systems, Minneapolis, United States, Cat. No.: RD-DMP900) plasma levels. Carbohydrate antigen-125 was measured by Liaison CA 125 II immunochemiluminescence assay (Cat. No.: 314211) on a Liaison Analyzer (DiaSorin, Saluggia, Italy).
Echocardiographic measurements were carried out by a licensed echocardiographic expert using a Phillips iE 33 system. Left ventricular ejection fraction was calculated using the Simpson biplane method, and left ventricular end-diastolic and end-systolic volumes were calculated using the Teicholz method. To demonstrate the reproducibility of echocardiographic measurements, we determined the interobserver and intraobserver variability with Lin's concordance correlation coefficient using 12-12 pairs of sample data, which proved substantial correlation (interobserver variability: ρc=0.956 [0.89-0.98]; intraobserver variability: ρc=0.96 [0.89-0.97]).
The data are expressed as the median [interquartile range] or as percentages with the event number. Continuous variables were compared using the Wilcoxon matched pair test and the Mann-Whitney test, as appropriate. For categorical data comparisons, the chi-square test was applied. The univariate logistic and Cox regression analyses were used to determine the baseline predictors of reverse remodelling and 5-year mortality. The continuous variables were standardized by 1 standard deviation increase. Receiver operating characteristic analysis was used, and we dichotomized the continuous variables and then compared the Kaplan-Meier curves by using log-rank tests. In the multivariable Cox regression models, the baseline model included variables with P<.05 value from the univariate analysis, and further adjusted models were built in a forward stepwise manner. Finally, we carried out reclassification analyses including c-statistics, net reclassification improvement (NRI), and integrated discrimination improvement (IDI).12,13
A 2-tailed P value of<.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS 22 (Apache Software Foundation, United States), GraphPad Prism 6.03 (GraphPad Software, Inc., United States) and PASS 2008 (NCSS, United States) the open source R software (R version 3.1.2 with PredictABEL and pROC packages).
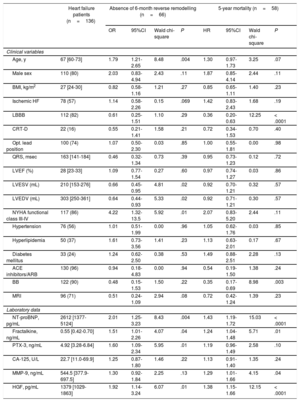
RESULTSStudy Population, Effects of Cardiac Resynchronization Therapy on Echocardiography Results and BiomarkersThe median age of the patients was 67 [60-73] years, and 80% of them were male. The median QRS duration was 163 [141-184] msec, and 82% of the patients had left bundle branch block (left bundle branch block) morphology in the electrocardiogram (Table 1). After 6 months of CRT, the left ventricular ejection fraction increased significantly 28% (23-33) vs 37% (31-41); P<.0001, while the left ventricular end-diastolic volume and left ventricular end-systolic volume decreased (left ventricular end-diastolic volume: 303 [251-351] mL vs 259 [203-332] mL; P<.0001; left ventricular end-systolic volume: 303 [154-276] vs 167 [116-242] mL; P<.0001).
Baseline Patient Characteristics as Predictors of Absence of 6-month Reverse Remodelling and 5-year Mortality
| Heart failure patients (n=136) | Absence of 6-month reverse remodelling (n=66) | 5-year mortality (n=58) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | Wald chi-square | P | HR | 95%CI | Wald chi-square | P | ||
| Clinical variables | |||||||||
| Age, y | 67 [60-73] | 1.79 | 1.21-2.65 | 8.48 | .004 | 1.30 | 0.97-1.73 | 3.25 | .07 |
| Male sex | 110 (80) | 2.03 | 0.83-4.94 | 2.43 | .11 | 1.87 | 0.85-4.14 | 2.44 | .11 |
| BMI, kg/m2 | 27 [24-30] | 0.82 | 0.58-1.16 | 1.21 | .27 | 0.85 | 0.65-1.11 | 1.40 | .23 |
| Ischemic HF | 78 (57) | 1.14 | 0.58-2.26 | 0.15 | .069 | 1.42 | 0.83-2.43 | 1.68 | .19 |
| LBBB | 112 (82) | 0.61 | 0.25-1.51 | 1.10 | .29 | 0.36 | 0.20-0.63 | 12.25 | < .0001 |
| CRT-D | 22 (16) | 0.55 | 0.21-1.41 | 1.58 | .21 | 0.72 | 0.34-1.53 | 0.70 | .40 |
| Opt. lead position | 100 (74) | 1.07 | 0.50-2.30 | 0.03 | .85 | 1.00 | 0.55-1.81 | 0.00 | .98 |
| QRS, msec | 163 [141-184] | 0.46 | 0.32-1.34 | 0.73 | .39 | 0.95 | 0.73-1.23 | 0.12 | .72 |
| LVEF (%) | 28 [23-33] | 1.09 | 0.77-1.54 | 0.27 | .60 | 0.97 | 0.74-1.27 | 0.03 | .86 |
| LVESV (mL) | 210 [153-276] | 0.66 | 0.45-0.95 | 4.81 | .02 | 0.92 | 0.70-1.21 | 0.32 | .57 |
| LVEDV (mL) | 303 [250-361] | 0.64 | 0.44-0.93 | 5.33 | .02 | 0.92 | 0.71-1.21 | 0.30 | .57 |
| NYHA functional class III-IV | 117 (86) | 4.22 | 1.32-13.5 | 5.92 | .01 | 2.07 | 0.83-5.20 | 2.44 | .11 |
| Hypertension | 76 (56) | 1.01 | 0.51-1.99 | 0.00 | .96 | 1.05 | 0.62-1.76 | 0.03 | .85 |
| Hyperlipidemia | 50 (37) | 1.61 | 0.73-3.56 | 1.41 | .23 | 1.13 | 0.63-2.01 | 0.17 | .67 |
| Diabetes mellitus | 33 (24) | 1.24 | 0.62-2.50 | 0.38 | .53 | 1.49 | 0.88-2.51 | 2.28 | .13 |
| ACE inhibitors/ARB | 130 (96) | 0.94 | 0.18-4.83 | 0.00 | .94 | 0.54 | 0.19-1.50 | 1.38 | .24 |
| BB | 122 (90) | 0.48 | 0.15-1.53 | 1.50 | .22 | 0.35 | 0.17-0.69 | 8.98 | .003 |
| MRI | 96 (71) | 0.51 | 0.24-1.09 | 2.94 | .08 | 0.72 | 0.42-1.24 | 1.39 | .23 |
| Laboratory data | |||||||||
| NT-proBNP, pg/mL | 2612 [1377-5124] | 2.01 | 1.25-3.23 | 8.43 | .004 | 1.43 | 1.19-1.72 | 15.03 | < .0001 |
| Fractalkine, ng/mL | 0.55 [0.42-0.70] | 1.51 | 1.01-2.26 | 4.07 | .04 | 1.24 | 1.04-1.48 | 5.71 | .01 |
| PTX-3, ng/mL | 4.92 [3.28-6.84] | 1.60 | 1.09-2.34 | 5.95 | .01 | 1.19 | 0.96-1.49 | 2.58 | .10 |
| CA-125, U/L | 22.7 [11.0-69.9] | 1.25 | 0.87-1.80 | 1.46 | .22 | 1.13 | 0.91-1.40 | 1.35 | .24 |
| MMP-9, ng/mL | 544.5 [377.9-697.5] | 1.30 | 0.92-1.84 | 2.25 | .13 | 1.29 | 1.01-1.66 | 4.15 | .04 |
| HGF, pg/mL | 1379 [1029-1863] | 1.92 | 1.14-3.24 | 6.07 | .01 | 1.38 | 1.15-1.66 | 12.15 | < .0001 |
95%CI, 95% confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BB, beta-blocker; BMI, body mass index; CA-125, carbohydrate antigen 125; CRT-D, cardiac resynchronization therapy with implantable cardioverter defibrillator; HF, heart failure; HGF, hepatocyte growth factor; HR, hazard ratio; Ischemic, ischemic etiology of heart failure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MMP-9, matrix metalloproteinase 9; MRI, mineralocorticoid receptor inhibitor; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; Opt. lead position, lateral or postero-lateral left ventricular lead position; OR, odds ratio; PTX-3, pentraxin 3.
Data are expressed as median [interquartile range] for continuous variables and as event numbers with percentage for categorical variables. The absence of 6-month reverse remodelling (absence of ≥ 15% decrease in the LVESV) was tested using univariate logistic regression analysis and the 5-year mortality was assessed using univariate Cox regression analysis. Continuous variables were standardized by 1 standard deviation increase. The OR and HR refer to presence vs absence for categorical variables and 1 standard deviation increase for continuous variables.
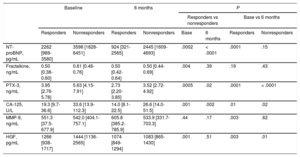
We investigated the change of the studied biomarkers during the 6 months of the follow-up period. We measured statistically significant lower plasma levels of pentraxin-3 (4.92 [3.29-6.84] ng/mL vs 3.13 [2.39-4.48] ng/mL; P<.0001), NT-proBNP (2612 [1377-5124] pg/mL vs 1626 [725-3300] pg/mL; P<.001), HGF (1379 [1029-1863] pg/mL vs 1083 [862-1328] pg/mL; P<.001) and carbohydrate antigen-125 (22.75 [11.05-69.90] U/L vs 18.30 [9.15-34.35]; P < .001) after 6 months of follow-up. Plasma levels of fractalkine and matrix metalloproteinase-9 remained statistically unaltered.
Association of Biomarker Concentrations With Clinical ResponseA total of 70 patients (51%) were considered as responders, and 66 patients (49%) were nonresponders according to our criteria (> 15% relative decrease in left ventricular end-systolic volume). N-terminal pro-B-type natriuretic peptide levels were significantly higher in nonresponders both at baseline (P=.0002) and at 6 months (P < .0001). We observed a significant decrease in plasma NT-proBNP levels only in responders (P=.0001). Pentraxin-3 and carbohydrate antigen-125 levels were considerably higher in nonresponders at baseline (pentraxin-3: P=.0005; carbohydrate antigen-125: P=.001) and 6 months (pentraxin-3: P=.02; carbohydrate antigen-125: P=.002). After CRT, plasma levels decreased both in responders (pentraxin-3: P=.0001; carbohydrate antigen-125: P=.01) and nonresponders (pentraxin-3: P < .0001; carbohydrate antigen-125: P=.02). Patients lacking a reverse remodelling response to CRT had significantly higher baseline HGF (P=.001) and fractalkine (P=.004) levels. Hepatocyte growth factor levels were significantly reduced following CRT in both groups (responders, P=.003; nonresponders, P=.01), while plasma fractalkine levels did not change over time. We found no significant differences in matrix metalloproteinase-9 levels between the groups, although we observed a significant rise in responders after 6 months (P=.003). The aforementioned results are detailed in Table 2.
Changes in Novel Heart Failure Biomarkers in Responders and Nonresponders (Responders Are Patients Who Underwent Reverse Remodelling 6 Months After CRT Implantation)
| Baseline | 6 months | P | ||||||
|---|---|---|---|---|---|---|---|---|
| Responders vs nonresponders | Base vs 6 months | |||||||
| Responders | Nonresponders | Responders | Nonresponders | Base | 6 months | Responders | Nonresponders | |
| NT-proBNP, pg/mL | 2262 [989-3580] | 3598 [1828-6451] | 924 [321-2565] | 2445 [1609-4693] | .0002 | < .0001 | .0001 | .15 |
| Fractalkine, ng/mL | 0.50 [0.38-0.60] | 0.61 [0.46-0.76] | 0.50 [0.42-0.64] | 0.50 [0.44-0.69] | .004 | .39 | .19 | .43 |
| PTX-3, ng/mL | 3.95 [2.76-5.78] | 5.63 [4.15-7.91] | 2.73 [2.20-3.85] | 3.52 [2.72-4.92] | .0005 | .02 | .0001 | < .0001 |
| CA-125, U/L | 19.3 [9.7-36.6] | 33.6 [13.9-112.3] | 14.0 [8.1-22.5] | 26.6 [14.0-51.5] | .001 | .002 | .01 | .02 |
| MMP-9, ng/mL | 551.3 [37.5-677.9] | 542.0 [404.1-757.1] | 605.8 [385.2-785.9] | 533.9 [331.7-703.3] | .44 | .17 | .003 | .62 |
| HGF, pg/mL | 1266 [938-1717] | 1444 [1136-2565] | 1074 [849-1294] | 1083 [865-1430] | .001 | .51 | .003 | .01 |
CA-125, carbohydrate antigen 125; CRT, cardiac resynchronization therapy; HGF, hepatocyte growth factor; LVESV, left ventricular end-systolic volume; MMP-9, matrix metalloproteinase 9; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PTX-3, pentraxin 3.
Data are expressed as median with interquartile range.
Reverse remodelling was defined as ≥ 15% decrease in the LVESV 6 months after CRT implantation.
Mann-Whitney and Wilcoxon tests were used for comparison.
Responders (n = 70); nonresponders (n = 66).
We investigated 5-year all-cause mortality by univariate Cox regression and 6-month reverse remodelling using univariate logistic regression analysis (Table 1). During the 5 years of follow-up, 58 patients (43%) died. All-cause mortality was significantly related to the absence of left bundle branch block (P < .0001), or beta-blocker therapy (P=.003) and increasing NT-proBNP levels (P < .0001), while increasing age showed a marginally significant association (P=.07). Of the novel biomarkers, increasing levels of fractalkine (P=.01), HGF (P < .0001) and matrix metalloproteinase-9 (P=.04) predicted 5-year mortality.
A total of 70 patients (51%) experienced reverse remodelling at 6 months based on our echocardiographic criterion. Increasing age (P=.004), NYHA functional class III-IV (P=.01) and increasing levels of NT-proBNP (P=.004) were predictive of the absence of 6-month reverse remodelling. A marginally significant relationship was seen between ischemic etiology of HF (P=.069), mineralocorticoid receptor inhibitor therapy (P=.08) and a lack of response. Increasing plasma levels of fractalkine (P=.04), pentraxin-3 (P=.01) and HGF (P=.01) predicted the lack of response to CRT.
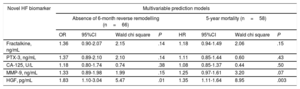
Multivariable Risk Prediction Models of Mortality and Reverse RemodellingTo further assess the impact of novel biomarkers on mortality, we set up a basic multivariable Cox regression model with all the variables previously shown to be relevant by the univariate analysis. The baseline multivariable model included age, left bundle branch block, beta-blocker therapy and NT-proBNP. In the next step, we entered the novel biomarkers separately into the baseline model in a forward stepwise manner. Only HGF predicted mortality in the multivariable model (HR, 1.35; 95%CI, 1.11-1.64; P=.003, per 1 standard deviation increase) (Table 3).
Multivariable Prediction Models for Each Biomarker, Including all Significant Baseline Parameters in the Basic Model
| Novel HF biomarker | Multivariable prediction models | |||||||
|---|---|---|---|---|---|---|---|---|
| Absence of 6-month reverse remodelling (n=66) | 5-year mortality (n=58) | |||||||
| OR | 95%CI | Wald chi square | P | HR | 95%CI | Wald chi square | P | |
| Fractalkine, ng/mL | 1.36 | 0.90-2.07 | 2.15 | .14 | 1.18 | 0.94-1.49 | 2.06 | .15 |
| PTX-3, ng/mL | 1.37 | 0.89-2.10 | 2.10 | .14 | 1.11 | 0.85-1.44 | 0.60 | .43 |
| CA-125, U/L | 1.18 | 0.80-1.74 | 0.74 | .38 | 1.08 | 0.85-1.37 | 0.44 | .50 |
| MMP-9, ng/mL | 1.33 | 0.89-1.98 | 1.99 | .15 | 1.25 | 0.97-1.61 | 3.20 | .07 |
| HGF, pg/mL | 1.83 | 1.10-3.04 | 5.47 | .01 | 1.35 | 1.11-1.64 | 8.95 | .003 |
95%CI, 95% confidence interval; CA-125, carbohydrate antigen 125; HF, heart failure; HGF, hepatocyte growth factor; HR, hazard ratio; MMP-9, matrix metalloproteinase 9; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OR, odds ratio; PTX-3, pentraxin 3.
Six-month reverse remodelling (n=70) was defined as at least a 15% decrease in end-systolic volume. The basic model for the multivariable logistic regression analysis included age, ischemic etiology, New York Heart Association functional class III-IV, mineralocorticoid receptor inhibitor, and NT-proBNP. The basic model for 5-year mortality (n=58) using multivariable Cox regression analysis included age, left bundle branch block, beta-blocker therapy, and NT-proBNP. In a forward stepwise manner, we adjusted the novel biomarkers to the basic models. The OR and HR refer to 1 standard deviation increase.
We applied the same approach to investigate reverse remodelling. We included all the relevant factors to the basic multivariable model: age, ischemic etiology of HF, NYHA functional class III-IV, mineralocorticoid receptor inhibitor therapy and NT-proBNP. We entered the novel biomarkers separately into the baseline model in a forward stepwise manner. Similarly to the mortality prediction, HGF was the only independent predictor of reverse remodelling (OR, 1.83; 95%CI, 1.10-3.04; P=.01, per 1 standard deviation increase) (Table 3).
Assessment of the Additional Benefit of Hepatocyte Growth Factor in the Prediction of OutcomeThe area under the curves were assessed by the receiver operating characteristics analysis to set up the best fitting cutoff values. Our aim was to determine a clinically relevant cut-off point that had relatively high sensitivity and the utmost specificity.
In this way, we found plasma HGF levels above 1236 pg/mL to be an optimal cutoff value (area under the curve=0.71; 95%CI, 0.63-0.80; P < .0001; sensitivity: 81% [69-90]; specificity: 54% [45-65]) (Figure 1). Hepatocyte growth factor levels exceeding 1236 pg/mL were associated with an increased risk of long-term mortality (HR, 3.48; 95%CI, 1.80-6.73; P < .0001) (Figure 1).
Impact of baseline HGF levels on 5-year mortality. A: comparison of the Kaplan-Meier survival curves by log-rank test. The survival of patients with baseline plasma HGF levels below and above 1236 pg/mL was compared. B: receiver operating characteristics analysis was performed to determine the optimal cutoff point for baseline plasma HGF levels. AUC, area under the curve; HGF, hepatocyte growth factor; HR, hazard ratio; NPV, negative predictive value; PPV, positive predictive value.
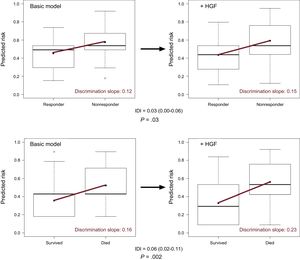
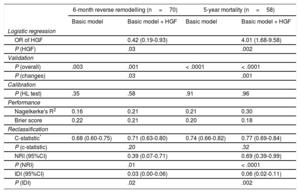
When HGF was added to the baseline multivariable model of mortality (NRI=0.69; 95%CI, 0.39-0.99; P < .0001; IDI=0.06; 95%CI, 0.02-0.11) and reverse remodelling (NRI=0.39; 95%CI, 0.07-0.71; P=.01; IDI=0.03; 95%CI, 0.00-0.06) respectively, we observed a significant improvement in reclassification and discrimination (Figure 2). Adding the HGF biomarker to the baseline model increased the discrimination slope of the prediction models (from 0.12 to 0.15 for reverse remodelling; from 0.16 to 0.23 for mortality). The predicted and observed risks, assessed by the Hosmer-Lemeshow test, did not differ significantly throughout the analyses, which indicated good calibration and confirmed the validity of the results (Table 4). The decrease in Brier score and increase in Nagelkerke's R2 suggested an improved predictive capacity.
Improved discrimination capacity of the prediction models. The improved discrimination capacity is visualized by means of a box and whiskers diagram. The discrimination slope is defined as the difference in the mean probabilities of events minus nonevents (upper panel, 6-month reverse remodelling; lower panel, 5-year mortality). Adding the HGF biomarker to the basic model increases the discrimination slope (red line) of the prediction models. The difference in the discrimination slopes is the IDI itself. HGF, hepatocyte growth factor; IDI, integrated discrimination improvement.
Validation, Calibration, and Reclassification Analyses
| 6-month reverse remodelling (n=70) | 5-year mortality (n=58) | |||
|---|---|---|---|---|
| Basic model | Basic model + HGF | Basic model | Basic model + HGF | |
| Logistic regression | ||||
| OR of HGF | 0.42 (0.19-0.93) | 4.01 (1.68-9.58) | ||
| P (HGF) | .03 | .002 | ||
| Validation | ||||
| P (overall) | .003 | .001 | < .0001 | < .0001 |
| P (changes) | .03 | .001 | ||
| Calibration | ||||
| P (HL test) | .35 | .58 | .91 | .96 |
| Performance | ||||
| Nagelkerke's R2 | 0.16 | 0.21 | 0.21 | 0.30 |
| Brier score | 0.22 | 0.21 | 0.20 | 0.18 |
| Reclassification | ||||
| C-statistic* | 0.68 (0.60-0.75) | 0.71 (0.63-0.80) | 0.74 (0.66-0.82) | 0.77 (0.69-0.84) |
| P (c-statistic) | .20 | .32 | ||
| NRI (95%CI) | 0.39 (0.07-0.71) | 0.69 (0.39-0.99) | ||
| P (NRI) | .01 | < .0001 | ||
| IDI (95%CI) | 0.03 (0.00-0.06) | 0.06 (0.02-0.11) | ||
| P (IDI) | .02 | .002 | ||
95%CI, 95% confidence interval; HGF, hepatocyte growth factor; HL, Hosmer-Lemeshow; NRI, net reclassification improvement; IDI, integrated discrimination improvement; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OR, odds ratio.
The basic multivariable logistic regression model for reverse remodelling included age > 75 years, ischemic etiology, New York Heart Association functional class III-IV, mineralocorticoid receptor inhibitor, and NT-proBNP > 1919 ng/mL, whereas the basic model for 5-year mortality involved age > 75 years, left bundle branch block, beta-blocker, and NT-proBNP > 1919 ng/mL.
HGF > 1236 ng/mL was adjusted to the basic models in a forward stepwise way.
In accordance with the criteria of the American Heart Association for evaluation of novel markers of cardiovascular risk, we displayed the measures of both discrimination accuracy and incremental information. Discrimination could be assessed via the c-statistic, which measures the differences of the total area under the curves of the prediction models by using the DeLong test (ie, P [c-statistic]). Accuracy is characterized by the calibration and performance of the prediction models. Calibration investigates if the observed risk (ie, number of death events of reverse remodelling) would differ from the calculated/predicted risk. The statistically nonsignificant (P ≥ .05) result of the HL test suggests that the observed and predicted risk correlates and therefore that the prediction model is well calibrated. The performance of the prediction model is displayed via the changes of the Nagelkerke R2 and the Brier score. The performance improves if the addition of the biomarker to the basic model results in increases in the Nagelkerke R2 (from 0.00 to 1.00) and simultaneous decreases in Brier score (from 0.25 to 0.00). The incremental value of novel markers could be presented via novel reclassification and discrimination methods. The NRI indicates how the total performance, the robustness of the basic model, improves by the addition of the novel marker. The discrimination could be assessed by the c-statistic, but this is a rather conservative method, which frequently underestimates the effect of relevant factors, and therefore the integrated discrimination improvement has been recently recommended to be applied. The IDI displays the differences of the discrimination slopes between the models (basic model vs basic model + HGF). The discrimination slope is the difference of the median risk of events and nonevents (ie, mortality vs survival; response vs nonresponse) predicted by the biomarker.
The main strength of our clinical study is that we investigated a set of promising HF biomarkers that has been previously shown to have prognostic information in various groups of HF patients. This is the first study to investigate the potential predictive value of these biomarkers in a cohort of CRT patients. The long-term follow-up period of 5 years provided a sufficient number of endpoints for analysis, even though the sample size was relatively small. Reclassification analyses allowed us to illustrate that HGF not only predicts both the response to CRT and long-term mortality, but also has an additive prognostic value to the relevant baseline confounders and the gold standard, NT-proBNP.
Possible Mechanisms and ExplanationChronic HF is a complex clinical syndrome affected by various pathophysiological processes. The exact role of inflammation, fibrosis, and neurohormonal activation in the development and progression of HF is under extensive research; nevertheless, all of them are under consideration as integrant components of the pathomechanism. As a consequence, numerous biomarkers have been studied, which reflects the activation of these processes and may enable diagnosis, therapy guidance, and prognosis assessment.14
Despite all the advances in the complex treatment of HF seen in the previous decades, morbidity and mortality are still high, and they exert a significant burden on health care worldwide.15 To address these problems, refinements in patient selection have been required, but approximately one-third of patients still do not respond to CRT.2 Thus, biomarkers predicting response or mortality may therefore be helpful in identifying high-risk patients in need of special care.
In our study, we investigated a set of novel HF biomarkers associated with different pathways of HF progression. These biomarkers have been shown to correlate with prognosis and HF severity in non-CRT populations.4–7,16 Levels of fractalkine, a mediator of inflammation, are elevated in HF, and this is independently associated with increased mortality.7 Pentraxin-3 is an important component of humoral innate immunity and has been associated with clinical outcomes and disease severity in HF patients with reduced and preserved ejection fraction.6 The tumor marker carbohydrate antigen-125 is used for screening, diagnosis and monitoring the treatment of ovarian cancer, and elevated levels were found in various types of malignancies, ascites, pleural effusion, and other types of peripheral edema. Furthermore, it reflects the hemodynamic status of both sides of the heart and correlates with fluid overload and clinical outcomes in HF patients.4,17 Matrix metalloproteinase-9 plays a leading role in myocardial remodelling by degrading extracellular matrix proteins. Along with other parameters, it was found to have predictive value in ischemic and dilated cardiomyopathy.5,8
Hepatocyte growth factor, a classic growth factor produced by a wide range of cell types, exerts angiogenetic, antifibrotic and antiapoptotic effects3; therefore, it could be considered a multipotent cardioprotective agent. This protective role was justified in a wide range of animal models using different methods of gene transfer.3,18 Elevated levels of HGF were found in acute and chronic HF patients, and surprisingly, this was a predictor of worse clinical outcomes.16,19 Taking these findings into consideration, highly elevated HGF levels may reflect the failing of the protective pathways of the cardiovascular system, therefore identifying patients in the most unstable clinical condition and at the highest risk.
A considerable favorable effect of CRT on outcomes was observed during follow-up, but clinical outcomes were slightly worse than those reported in the literature. This finding can be explained by the fact that in the Hungarian population overall, cardiovascular mortality is higher and life expectancy is significantly shorter than in high-income countries.20
In this study, pentraxin-3 predicted reverse remodelling and matrix metalloproteinase-9 predicted long-term mortality, while fractalkine was a predictor of both endpoints in univariate models. Despite these promising results, all those previous biomarkers lost their predictive values in the multivariate models. Hepatocyte growth factor was the only marker that was able to predict both the absence of reverse remodelling and 5-year mortality independently of other clinical covariates.
Furthermore, we analyzed the predictive value of HGF by reclassification analyses, which showed statistically significant reclassification improvement and discrimination development in the case of both endpoints, demonstrating that measuring HGF may be beneficial in the risk assessment of HF patients undergoing CRT. This result parallels the findings of Richter et al.,21 who found HGF to be the strongest predictor of cardiovascular mortality in advanced HF patients among several novel HF biomarkers. A possible explanation could be that HGF is a growth factor that acts directly on the level of genes and is triggered by nonspecific cardiovascular distress. Hence, HGF reveals a more comprehensive picture of the complex HF pathophysiology than the other biomarkers, which instead reflect a particular pathway.
Based on our results, HGF may be helpful in identifying CRT patients with the highest risk of poor clinical outcomes. Patients with inadequate response to CRT need special care and early consideration of heart transplantation or mechanical circulatory support. Due to the relevant predictive value of HGF, it may be useful for the timely identification of potential nonresponders or it may be used for guiding further device therapy in the absence of clinical response to CRT.
LimitationsNotable limitations are the single-center design and the relatively small sample size. We considered all-cause mortality as the primary endpoint; cardiovascular death was not investigated separately due to the relatively low event numbers. Routine laboratory measurement of HGF is not widespread to date, which might limit the direct adaptation of our findings into real-world clinical practice. Left ventricular end-systolic volumes were measured using the Teicholz method, and not with the Simpson method, which would have been more accurate for calculating LV volumes in this particular patient cohort. Taking all these factors into consideration, our results should be interpreted as preliminary hypothesis-generating findings. Their validation is clearly necessary with larger, prospective studies.
CONCLUSIONSIncreased HGF levels predict the absence of reverse remodelling and long-term mortality in HF patients undergoing CRT independently of NT-proBNP or other relevant factors. Reclassification analyses showed that adding HGF resulted in improved prediction performance, discrimination and reclassification in the prediction models. Thus, HGF may be helpful in patient selection for CRT and in identifying patients at highest risk of mortality or insufficient clinical response.
FUNDINGThis scientific work was supported by the Hungarian Foundation Programs “Semmelweis Egyetem Híd Projekt” (TÁMOP-4.2.2-08/1/KMR-2008-0004) and “Semmelweis Egyetem Magiszter Program” (TÁMOP-4.2.2./B10/1.-210-0013), János Bolyai Research Scholarships of the Hungarian Academy of Sciences (G. Széplaki and L. Gellér), and the Hungarian Scientific Research Fund (OTKA K 105555).
CONFLICTS OF INTERESTNone declared.
- –
The novel HF biomarkers fractalkine, pentraxin-3, HGF, carbohydrate antigen-125 and matrix metalloproteinase-9 are associated with clinical response in acute and chronic HF, but there are no previous data on their usefulness in predicting outcomes after CRT.
- –
Our results demonstrate that HGF predicts 5-year mortality and the absence of 6-month reverse remodelling in HF patients undergoing CRT, independently of NT-proBNP and other baseline predictors of outcome. Patients with baseline HGF levels exceeding 1236 pg/mL have a more than 3-fold increased 5-year mortality risk. Reclassification analyses revealed that applying HGF to the predictive model significantly improved risk stratification.
The authors acknowledge the laboratory assistance of Éva Fórizs.