It is well known that the apparent significant coronary stenosis on angiography sometimes does not cause significant ischemia, and vice versa. For this reason, decision-making based on coronary physiology is becoming more and more important. Fractional flow reserve (FFR), which has emerged as a useful tool to determine which lesions need revascularization in the catheterization laboratory, now has a class IA indication in the European Society of Cardiology guidelines. More recently, the instantaneous wave-free ratio, which is considered easier to use than FFR, has been graded as equivalent to FFR. This review discusses the concepts of FFR and instantaneous wave-free ratio, current evidence supporting their use, and future directions in coronary physiology.
Keywords
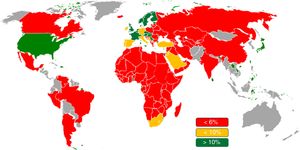
Coronary physiology has established its position in the field of catheter intervention. Fractional flow reserve (FFR) has enabled us to manage patients with coronary artery disease (CAD) more accurately and safely than by angiographical visual estimation. Currently, FFR is widely used worldwide (Figure 1). Because abundant evidence including 3 major randomized trials (DEFER, FAME, and FAME 2 trial1–3) has been amassed for over 20 years, use of FFR for stable CAD is recommended by the guidelines of the ESC/EACTS (European Society of Cardiology/European Association for Cardio-Thoracic Surgery) and the ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS (American College of Cardiology/American Association for Thoracic Surgery/American Heart Association/American Society of Echocardiography/American Society of Nuclear Cardiology/Society for Cardiovascular Angiography and Interventions/Society of Cardiovascular Computed Tomography/Society of Thoracic Surgeons).4–6 Recently, the instantaneous wave-free ratio (iFR) has been introduced as an alternative to FFR, which does not require hyperemia.7 Five years after the initial introduction, 2 large randomized trials, DEFINE-FLAIR and iFR-SWEDEHEART trial, have simultaneously reported the noninferiority of iFR compared with FFR.8,9 Currently, iFR is considered equivalent as a diagnostic modality for ischemic heart disease in the latest guidelines.6 In this review article, we summarize the evidence of coronary physiology, especially concerning these 2 modalities, and we also discuss future directions in this field.
Fractional flow reserve use in different parts of the world in 2016. Fractional flow reserve is currently used worldwide in decision-making. However, the frequency of its use in the catheterization laboratory is not high, despite clinical guideline recommendations. Reproduced with permission from Philips Volcano, market research report by Decision Resources Group.
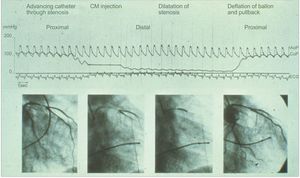
Forty years ago, the world's first percutaneous coronary intervention (PCI) was performed by Andreas Grüntzig.10 In fact, coronary pressure across the stenosis before and after PCI was recorded for the first time at the same time (Figure 2). Grüntzig measured the trans-stenotic pressure gradient through the fluid-filled guiding catheter. Despite early introduction to the field of PCI, practical use of coronary physiology in the catheterization laboratory did not begin until the late 1990s because of technological and theoretical aspects. Because the currently used well-matured pressure wires were not available, coronary pressure was measured for years by the catheter itself, which was found to be not very reliable due to the impaired antegrade flow induced by catheter engagement.11 The relationship between coronary pressure and coronary flow was actively investigated after the notion of hyperemia was introduced.12 Finally, technological and theoretical advances allowed the development of the concept of FFR.
First case of percutaneous coronary intervention. In 1979, coronary pressure across the stenosis before and after balloon angioplasty was recorded in the first case of coronary intervention. AoP, aortic pressure; CM, contrast media; CoP, coronary pressure. Reproduced with permission from Grüntzig et al.10
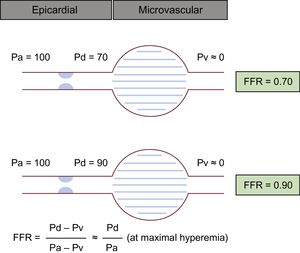
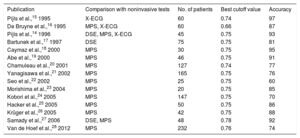
FFR was first described by Pijls et al. in 1993.13 The concept of FFR is based on the notion that maximal hyperemia could achieve a linear correlation between coronary flow and coronary pressure because coronary resistance is stable and minimal during maximal arterial dilation. FFR is defined as the ratio of mean distal pressure (Pd) relative to mean aortic pressure (Pa) during maximal hyperemia induced by vasodilating agents. For example, an FFR of 0.70 means that coronary flow is reduced by 30% from normal and an FFR of 0.90 means that coronary flow is reduced by only 10% from normal due to the existence of stenoses (Figure 3). Taking into account stenosis severity, myocardial territory and viability, and collateral perfusion, FFR is able to assess the functional significance of CAD. The cutoff value to detect significant ischemia was suggested to be 0.75 by a relatively small study, which demonstrated high diagnostic performance of FFR, with a sensitivity of 88%, specificity of 100% and accuracy of 93% compared with dobutamine stress echocardiography, stress myocardial perfusion scintigraphy and exercise stress electrocardiography.14 ¿Subsequently, numerous studies were conducted to compare FFR with noninvasive functional testing and found an acceptable overall correlation15–28 (Table 1). Nowadays, the best cutoff value to defer PCI in clinical practice is considered to be 0.80 after validation in multiple prospective, randomized trials with this threshold.2,3
Schema for the concept of FFR. During maximal hyperemia, a direct relationship between coronary pressure and flow could be presumed because the effect from microvascular could be ignored. Despite the same degree of stenoses on visual estimation, the illustrated cases show totally different FFR values. FFR, fractional flow reserve; Pa, aortic pressure; Pd, distal pressure; Pv, central venous pressure.
Studies of Fractional Flow Reserve Compared With Noninvasive Functional Testing
| Publication | Comparison with noninvasive tests | No. of patients | Best cutoff value | Accuracy |
|---|---|---|---|---|
| Pijls et al.,15 1995 | X-ECG | 60 | 0.74 | 97 |
| De Bruyne et al.,16 1995 | MPS, X-ECG | 60 | 0.66 | 87 |
| Pijls et al.,14 1996 | DSE, MPS, X-ECG | 45 | 0.75 | 93 |
| Bartunek et al.,17 1997 | DSE | 75 | 0.75 | 81 |
| Caymaz et al.,18 2000 | MPS | 30 | 0.75 | 95 |
| Abe et al.,19 2000 | MPS | 46 | 0.75 | 91 |
| Chamuleau et al.,20 2001 | MPS | 127 | 0.74 | 77 |
| Yanagisawa et al.,21 2002 | MPS | 165 | 0.75 | 76 |
| Seo et al.,22 2002 | MPS | 25 | 0.75 | 60 |
| Morishima et al.,23 2004 | MPS | 20 | 0.75 | 85 |
| Kobori et al.,24 2005 | MPS | 147 | 0.75 | 70 |
| Hacker et al.,25 2005 | MPS | 50 | 0.75 | 86 |
| Krüger et al.,26 2005 | MPS | 42 | 0.75 | 88 |
| Samady et al.,27 2006 | DSE, MPS | 48 | 0.78 | 92 |
| Van de Hoef et al.,28 2012 | MPS | 232 | 0.76 | 74 |
DSE, dobutamine stress echocardiogram; MPS, myocardial perfusion scintigraphy; X-ECG, exercise electrocardiogram.
We summarize the 3 major randomized trials that played a very important role in establishing evidence of FFR:
DEFER StudyThe initial randomized DEFER study was conducted to evaluate the safety of deferring PCI guided by FFR.1 In that study, patients with stable angina and intermediate stenosis but FFR > 0.75 were randomized to deferral (deferral group) or performance (perform group). Subsequent to this original report, long and longer-term follow-up of the DEFER cohort is now available at 5 years29 and 15 years.30 Event-free survival did not differ between the deferral and performance groups. The authors concluded that patients with FFR > 0.75 were stable and safe and that stenting did not decrease the risk of cardiac events for CAD without significant ischemia.
FAME StudyThe second randomized control trial was larger than DEFER. The FAME study was performed to assess the effectiveness of FFR-guided PCI compared with angio-guided PCI in patients with multivessel CAD.2 In this trial, 1005 patients with at least 50% stenoses of the vessel diameter in at least 2 of the 3 major epicardial coronary arteries were randomly assigned to undergo PCI with drug-eluting stents (DES) guided by FFR measurements or guided by angiography alone. The cutoff value for decision-making was 0.80. The authors concluded that FFR-guided PCI significantly reduced the rate of the composite endpoint of death, nonfatal myocardial infarction, and repeat revascularization at 1 year (13.2% vs 18.3%; relative risk, 0.72; 95% confidence interval, 0.54 to 0.96, respectively; P = .02). After this study, the cutoff value of FFR 0.80 has been often used to judge whether to perform or defer PCI in clinical practice, although FFR 0.75 is the definitive cutoff to determine significant ischemia or not, which subsequently generated the problem of the “gray zone FFR”.
FAME 2 StudyThe third trial was the FAME 2 study to examine whether FFR-guided PCI plus optimal medical therapy (OMT) was superior to OMT alone or not.3 A 2-year clinical follow-up was planned for 1220 patients. However, the adverse event of urgent revascularization in the OMT alone group occurred more frequently than expected and the study was discontinued (mean follow-up: 7 months). The authors concluded that FFR-guided PCI plus OMT, as compared with OMT alone, decreased the need for urgent revascularization in patients with stable CAD with proven ischemia. Because of the discontinuance of the study, the investigators could not reach any conclusions regarding the hard endpoint of death or myocardial infarction.
CURRENT SITUATIONWith over 20 years of investigations and clinical experience, coronary physiology has established its place in the field of catheter intervention. Clinical outcomes during the 15-year follow-up of the DEFER study and the 5-year follow-up of the FAME study have strengthen the idea that FFR-guided decision-making is safe and rational.29,30 During this period, knowledge and evidence of FFR have encountered more specific problems in the various clinical settings. Some of them will be discussed in the first part of this section.
Usefulness of FFR in Real-world Clinical PracticeMultivessel DiseaseAs the DES-Era has started and newer-generation DES have become available, PCI is currently widely performed in multivessel disease. However, long-term clinical outcomes of PCI in 3-vessel disease is less satisfactory than expected despite newer DES use when revascularization is guided by angiography.31,32 On the other hand, in the FAME study, FFR-guided PCI in multivessel disease achieved better clinical outcomes, and additionally, showed that the number of stents used per patient was significantly lower in the FFR-guided group (1.9 ± 1.3 vs 2.7 ± 1.2; P < .001).2
Although myocardial perfusion scintigraphy is considered the standard modality for detecting ischemia, several studies showed there was discordance in the results compared with FFR in multivessel disease.33,34 This discordance could be explained by the phenomenon of balanced ischemia, in which the nuclear stress test appears normal as a result of reduced myocardial perfusion in all coronary territories in patients with multivessel disease. Therefore, evaluation with FFR for multivessel disease is considered rational in decision-making for revascularization.
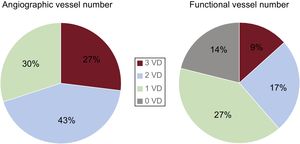
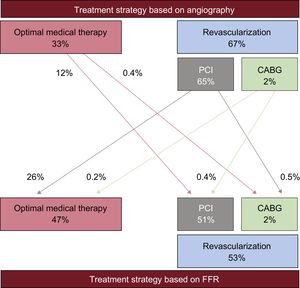
It is well known that physiological evaluation with FFR reduces the number of functionally diseased vessels and could also change patient management35,36 (Figure 4). Recently, a Japanese multicenter registry showed that reclassification of the treatment strategy was observed in approximately 40% of patients with CAD by FFR measurement37,38 (Figure 5).
Vessel number evaluated by angiography and fractional flow reserve. The number of functional diseased vessels can change markedly from the initial angiographic assessment. VD, vessel disease. Reproduced with permission from Sant’Anna et al.35
Results from the CVIT-DEFER registry. The treatment strategy can be changed drastically by functional assessment. CABG, coronary artery bypass graft; FFR, fractional flow reserve; PCI, percutaneous coronary intervention. Reproduced with permission from Nakamura et al.37,38
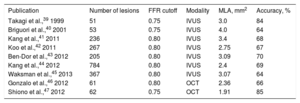
The relationship between FFR and intracoronary imaging modalities has been also evaluated.39–47 In the early day of this investigation, intravascular ultrasound (IVUS) showed good correlation with FFR.39 However, recent reports revealed that the accuracy to predict significant FFR by minimum lumen area (MLA) on IVUS was approximately 60% to 70%, which was considered unsatisfactory in clinical practice (Table 2). This discordance could be explained by the fact that evaluations with MLA alone do not consider the target vessel diameter, lesion lengths, and collateral flow.44,45 Optical coherence tomography has emerged as an invasive intracoronary imaging modality that provides better resolution than IVUS, offering more precise evaluation of the vessel lumen and stent edge.48 However, its correlation with significant FFR was also limited and the best cutoff values of MLA to predict significant FFR tended to be smaller than those of IVUS46,47 (Table 2). Thus, IVUS and optical coherence tomography could not be an alternative to FFR and complimentary use of intracoronary imaging modalities and functional assessment should be applied at present.
Correlation in Studies Between Fractional Flow Reserve and Imaging Modalities
| Publication | Number of lesions | FFR cutoff | Modality | MLA, mm2 | Accuracy, % |
|---|---|---|---|---|---|
| Takagi et al.,39 1999 | 51 | 0.75 | IVUS | 3.0 | 84 |
| Briguori et al.,40 2001 | 53 | 0.75 | IVUS | 4.0 | 64 |
| Kang et al.,41 2011 | 236 | 0.80 | IVUS | 3.4 | 68 |
| Koo et al.,42 2011 | 267 | 0.80 | IVUS | 2.75 | 67 |
| Ben-Dor et al.,43 2012 | 205 | 0.80 | IVUS | 3.09 | 70 |
| Kang et al.,44 2012 | 784 | 0.80 | IVUS | 2.4 | 69 |
| Waksman et al.,45 2013 | 367 | 0.80 | IVUS | 3.07 | 64 |
| Gonzalo et al.,46 2012 | 61 | 0.80 | OCT | 2.36 | 66 |
| Shiono et al.,47 2012 | 62 | 0.75 | OCT | 1.91 | 85 |
FFR, fractional flow reserve; IVUS, intravascular ultrasound; MLA, minimum lumen area; OCT, optical coherence tomography.
PCI of bifurcation lesion is still challenging, whereas FFR has provided useful knowledge in such cases. Koo et al.,49,50 used FFR to assess the jailed side-branch after crossover stenting in bifurcation lesions. Although angiographic stenosis was apparent in many of the cases, less than one-third of these ostial lesions with a stenosis diameter > 75% were found to have FFR < 0.75. The authors suggested that an additional intervention for the jailed side-branch was not necessary if coronary blood flow was preserved. Nowadays, this knowledge has been expanded to left main bifurcation lesions, which are one of the most challenging themes for PCI, supporting the feasibility of left main crossover stenting.51
Hyperemic AgentsSeveral hyperemic agents are available. Intravenous (IV) injection of adenosine, particularly via a central venous line, is the gold standard, and acts within 1 to 2minutes, creates a steady level of maximum hyperemia, and is relatively safe as drug-stress.52,53 During adenosine injection, patients often feel discomfort in the chest or the throat. Intracoronary (IC) injection of adenosine can be applied safely and other hyperemic agents, such as adenosine 5’-triphosphate (IV or IC), papaverine (IC) and nicorandil (IC) are available.54–56 The difference between each hyperemic agent is summarized in Table 3.
Properties of Several Hyperemic Agents
| Hyperemic agents | Injection type | Dose | Time to maximal hyperemia | Duration | Advantages | Adverse effects and other disadvantages* |
|---|---|---|---|---|---|---|
| Adenosine/ATP | IV | 140-150μg/kg/min | 2-3min | 1-2 min | Safe | Chest discomfort and face flush (30%-70%), blood pressure drop, AV block (very rare), contraindication for asthma |
| Adenosine/ATP | IC | LCA: ≥80μg RCA: ≥40μg | 5-10 sec | 5 sec | Quick response | AV block (rare) Does not allow pullback due to short duration* |
| Papaverine | IC | LCA:12-20 mg RCA: 8-12 mg | 15 sec | 30-60 sec | Quick response | QT time prolongation, torsade de pointes (0.5%) |
| Nicorandil | IC | 2 mg | 15 sec | 20-30 sec | Safe and quick response | VF (extremely rare) Expensive* |
AV block, atrioventricular block; ATP, adenosine 5’-riphosphate; IC, intracoronary injection; IV, intravenous injection; LCA, left coronary artery; RCA, right coronary artery; VF, ventricular fibrillation.
It is also known that the introduction of FFR to clinical practice makes economic sense since the FAME study demonstrated that FFR-guided PCI saved $675 per patient in procedure time and saved > $2000 per patient at 1 year while achieving preferable clinical outcomes.57 Most recently, the cost-effectiveness of FFR-guided PCI was shown by the 3-year follow-up of the FAME 2 trial, which demonstrated that PCI in patients with stable CAD and reduced FFR was advantageous compared with OMT alone because it resulted in improved clinical outcomes and quality of life at no increased cost at the 3-year follow-up.58
Limitations of FFRAlthough the use of FFR in clinical practice has many benefits, as shown, there are also some problems.
Low Performance RateDespite strong recommendation from clinical guidelines, FFR is not as frequently used as expected (Figure 1). The potential reasons for the low performance rate may include the time needed to take FFR measurements, the additional invasiveness of wiring in coronary angiography, the adverse effects of hyperemic agents, the costs associated with adenosine, patient-related discomfort, contraindications, and lack of reimbursement.59
Difference in Drug ResponseAlthough adenosine (IV) is the gold standard for hyperemia, previous studies revealed that there were various types of response to adenosine (IV), which might make it difficult to assess true FFR, separate from minimum Pd/Pa,60,61 and it is also known that some patients do not respond well to adenosine.
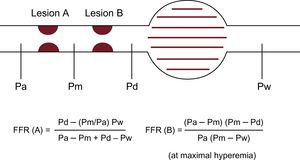
Tandem LesionsTandem lesions are defined as 2 separate lesions with > 50% stenosis each in the same coronary artery, separated by an angiographically normal segment. FFR in tandem lesions often misleads the interventional cardiologists because each stenosis will influence hyperemic blood flow and therefore an accurate pressure gradient due to each stenosis cannot be known before one is treated.62 Although equations for predicting the FFR of each individual lesion separately were suggested,63 they are not practical because wedge pressure measurement requires balloon inflation at the distal vessel (Figure 6). Practical intervention should be based on a step-by-step strategy: after the stenosis with the largest gradient has been stented, the pullback recording should be repeated to determine whether and where a second stent should be deployed,64 which seems time-consuming and bothersome for most of the interventional cardiologists.
Schema for the concept of FFR in tandem lesions. Equations for predicting FFR of each individual lesion separately is available but complicated. FFR, fractional flow reserve; Pa, aortic pressure; Pd, distal pressure; Pm, coronary pressure between 2 lesions; Pw, coronary wedge pressure.
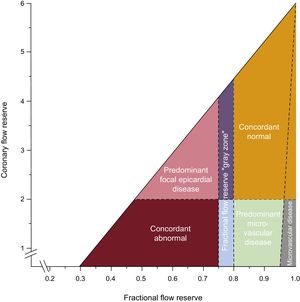
Although the diagnostic accuracy of FFR and coronary flow reserve (CFR) for ischemic heart disease is known to be equivalent, the results of FFR and CFR are discordant in 30% to 40% of CAD65: a phenomenon that has been proposed to originate from the different distribution of epicardial and microvascular involvement66 (Figure 7). The adverse outcome of discordance between FFR and CFR was demonstrated compared with cases in which FFR and CFR were normal, which was particularly attributable to those cases with normal FFR and abnormal CFR, whereas discordance with abnormal FFR and normal CFR was predominantly associated with equivalent clinical outcomes compared with concordantly normal FFR and CFR.67
Conceptual plot of the fractional flow reserve-coronary flow reserve relationship. There is discordance between them. Reproduced with permission from Van de Hoef et al.67
The best approach for the management of lesions with gray zone FFR, defined as an FFR value between 0.75 and 0.80, is unknown since previous studies have shown conflicting outcome data for these patients.68,69 It would be rational to make decisions based on multilateral clinical judgment for individual patients with gray zone FFR, including other perfusion imaging modalities, considering anatomical features, patient background, and the risk-benefit profile for PCI.
iFR-Era Just Around the CornerMore recently, interest in resting gradients has emerged, given the many limitations of FFR discussed above. Most notably, iFR was introduced as an alternative to FFR in 20127 and has shown equivalent diagnostic performance compared with FFR in several studies with invasive and noninvasive modalities in a very short time span.70–74 Additionally, iFR-guided PCI was noninferior to FFR-guided PCI for adverse cardiovascular events at 1-year follow-up in 2 separate, large randomized multicenter trials,8,9 which led to iFR being included in the clinical guidelines as equivalent to FFR in the United States.6
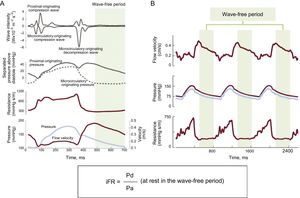
ConceptThe iFR is measured in the mid to late diastolic period of low and constant coronary resistance, called the wave-free period, and the mean pressure during the wave-free period is obtained distal to the lesion and indexed to simultaneous Pa.7 In this period, pressure and flow velocity are linearly related, allowing for the pressure-only index to evaluate the severity of the coronary lesion. Furthermore, isolation of the pressure measurement to the wave-free period eliminates the interaction between the myocardium and coronary microvasculature in systole and early diastole, during which the compression of the microvasculature increases intracoronary resistance75,76 (Figure 8).
Schema for the concept of wave-free period and iFR. A: The green shading highlights the wave-free period of diastole where the multiple different waves propagating from the proximal and distal ends of the vessel are quiescent. B: Flow velocity (top trace), proximal (light blue), and distal (purple) pressure traces and instantaneous resistance (bottom trace) demonstrate the stability of the wave-free period beat to beat. iFR, instantaneous wave-free ratio; Pa, aortic pressure; Pd, distal pressure. Reproduced with permission from Nijjer et al.76
Several studies after the introduction of iFR concluded that concordance with FFR was observed in approximately 80%, which also revealed that detection of the wave-free period was necessary in measurement.77–79 Following these comparisons of iFR with FFR, a series of further comparison studies were conducted between iFR, FFR, and third-party arbiters of ischemia (Table 4). These studies found not only equivalent diagnostic performance of iFR70–74 but also the possibility of a higher correlation between iFR and microvascular function compared with FFR.71,72
Comparison Between Instantaneous Wave-free Ratio and Fractional Flow Reserve in Ischemia Assessment
| Publication | Modality | No. | FFR diagnostic accuracy of AUC (%) | iFR diagnostic accuracy of AUC (%) | P |
|---|---|---|---|---|---|
| Sen et al.,70,2013 | HSR | 51 | 92 | 92 | NS |
| Sen et al.,71 2013 | HSR | 120 | 82 | 89 | < .01 |
| Petraco et al.,72 2014 | CFR | 216 | 67 | 74 | < .01 |
| Van de Hoef et al.,73 2015 | MPS | 85 | 63 | 62 | NS |
| Hwang et al.,74 2017 | PET | 115 | 70 | 74 | NS |
AUC, area under the curve; CFR, coronary flow reserve; FFR, fractional flow reserve; HSR, hyperemic stenosis resistance; iFR, instantaneous wave-free ratio; MPS, myocardial perfusion scintigraphy; NS, not significant; PET, positron emission tomography.
Most recently, the effectiveness of iFR to guide PCI compared with FFR was reported in DEFINE-FLAIR and iFR-SWEDEHEART, the largest randomized clinical trials in the field of coronary physiology to date.8,9 Over 4500 patients were randomized in 2 different studies in a 1:1 fashion to either iFR-guided or FFR-guided PCI, with iFR ≤ 0.89 and FFR ≤ 0.80 as cutoffs for revascularization. The primary endpoint, a composite of death from any cause, nonfatal myocardial infarction, or unplanned revascularization at 1 year, occurred in DEFINE-FLAIR and iFR-SWEDEHEART in 6.8% and 6.7% in the iFR groups and in 7.0% and 6.1% in the FFR groups, respectively. The investigators concluded that iFR-guided PCI was noninferior to FFR-guided PCI with respect to the risk of cardiovascular events at 1 year (Figure 9). In addition, in the iFR groups, the number of functionally significant stenoses and rates of revascularization were lower, the duration of the procedural time was shorter, and the percentage of patients who developed adverse symptoms related to the procedure was smaller.
Results of DEFINE-FLAIR and iFR-SWEDEHEART. Kaplan-Meier curves demonstrating noninferiority of iFR vs FFR for major adverse cardiac events at 12 months in both trials. 95%CI, 95% confidence interval; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio. Reproduced with permission from Davies et al.8 and Götberg et al.9
In current clinical practice, FFR is preferred because evidence has been generated for years, whereas iFR tends to be preferred because of additional advantages and emerging new evidence. Clinical guidelines have ranked them as equivalent.6 The recently published SYNTAX II study data showed that the application of coronary physiology to the PCI technique, regardless of FFR or iFR, could improve clinical outcomes for patients with 3-vessel disease.80
FUTURE DIRECTIONSCoronary Physiology in ACSOne of the current interests in this field is physiological interrogation of nonculprit lesions in acute coronary syndrome (ACS). Two recent randomized trials have supported revascularization of the noninfarct-related artery with FFR guidance. The DANAMI-3-PRIMULTI trial demonstrated that FFR-guided staged complete revascularization before hospital discharge led to significantly decreased future revascularizations at 1-year follow-up compared with PCI for the infarct-related artery only.81 Similarly, the Compare-Acute trial investigators examined whether FFR-guided treatment improved outcomes in patients with ST-segment elevation myocardial infarction and multivessel disease to evaluate the benefit of revascularization in the acute setting of noninfarct-related lesions.82 FFR -guided revascularization at the time of primary PCI resulted in a lower rate of the composite cardiovascular event rate at 1 year, mainly driven by decreased subsequent revascularizations. However, due to the lack of a physiological comparator arm, the full potential of coronary physiology applied to ACS could not be revealed, and should therefore be evaluated in future studies. In addition, it is important to note that invasive physiological assessment of the noninfarct-related artery in ACS is not a benign process. Serious adverse events occurred in 0.2% of the Compare-Acute trial population, including dissection of a coronary artery with subsequent vessel occlusion, myocardial infarction, and death. The risk-benefit balance of additional wiring to nonculprit lesions or inducing hyperemia in ACS is obscure and requires evaluation.
As Guidance for CABGEvidence for using coronary physiology to guide coronary artery bypass graft (CABG) is lacking. Only one study has shown that the rate of occlusion was significantly higher on angiography at 1 year when the bypass was placed on functionally nonsignificant stenoses, which suggested that coronary physiology could provide better clinical outcomes of CABG as well as in the field of PCI.83 Prospective, randomized trials focusing on hard clinical endpoints are needed to address the merits of FFR/iFR-guided CABG.
Potential of iFRRegarding the frequency of use, iFR will become more widely used when longer-term clinical outcomes are reported from the DEFINE-FLAIR and iFR-SWEDEHEART trials.
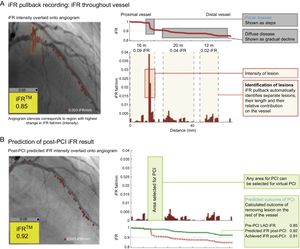
Because of the application of resting index, a recent report suggested that iFR could resolve one of the limitation of FFR: hemodynamic crosstalk between stenoses in tandem lesions during hyperemic status.63 Intracoronary pressure mapping, which allows identification of the segments that contribute most to hemodynamic impairment caused by diffuse or tandem lesions and prediction of post-iFR after virtual stenting, was demonstrated in the iFR pullback study84 (Figure 10). This technology is more advanced and should be validated in the near future. However, to use this technology maximally, improvement in the property of the pressure wire is mandatory so that the pressure wire can be used as a PCI guidewire in real-world interventions.
Representative case from the iFR pullback study. A: The coregistration of the iFR pullback trace with coronary angiography identifies pressure loss along the length of the vessel and distinguishes focal from diffuse disease. B: Virtual percutaneous coronary intervention (PCI) calculates the expected post-PCI iFR result for the area selected for PCI. iFR, instantaneous wave-free ratio; LAD, left anterior descending coronary artery. Reproduced with permission from Nijjer et al.84
Recently, the notion of computed fluid dynamics has contributed to combining coronary imaging with coronary physiology and has produced several promising modalities. Cardiac computed tomography-based FFR has achieved high diagnostic performance in several prospective multicenter studies compared with computed tomography angiography85–87 and has suggested advantages in quality of life and economic outcomes compared with conventional clinical practice.88 The quantitative flow ratio, which is a 3-dimensional quantitative coronary angiography-based FFR, could determine the hemodynamically significant lesion without pressure wire or hyperemia.89 Optical coherence tomography-derived virtual FFR might elucidate the relationship between coronary morphology and physiology.90 These technologies are aimed to indicate the simulated value of FFR from coronary imaging only, taking advantages of each modality's features.
CONCLUSIONSCoronary physiology is becoming increasingly important for current interventional cardiologists with abundant evidence and an evolving future. The evidence amassed to date would have to say “Use FFR/iFR for better PCI”. Growing novel technologies in this field are also attracting a great deal of interest.
CONFLICTS OF INTERESTT. Warisawa and C.M. Cook have received consulting fees from Philips. J.E. Davies has received research grants from Philips and AstraZeneca, consulting fees from Medtronic, Philips, and ReCor Medical, and holds patents pertaining to the iFR technology, which is under license to Philips.
.