Perivalvular complications of infective endocarditis (IE) include abscesses, pseudoneurysms, and fistulas. Surgical treatment is mandatory in these cases to prevent progression of heart failure resulting from irreversible structural valve damage. Despite the high rate of surgery in these patients (87%), in-hospital mortality remains high at around 41%.1,2
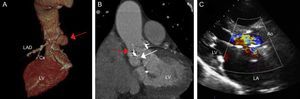
We present the case of an 86-year-old man who had undergone aortic valve replacement with a biological prosthetic valve (C-E Perimount Magna no. 23) 7 years earlier. He was hospitalized due to prosthetic valve IE and an abscess in the posterior region of the aortic annulus. Surgical treatment was proposed during discussion of the case but rejected due to the high surgical risk (EuroSCORE II = 25.47; Society of Thoracic Surgeons score = 27.70). The patient was discharged after completing a full antibiotic course, but his condition deteriorated with New York Heart Association functional class IV heart failure. Several imaging tests were performed, which confirmed the cavitation and fistulization of the abscess, causing severe aortic regurgitation with dilatation and progressive deterioration in left ventricular systolic function (Figure 1A-C). The abscess extended from the noncoronary leaflet, bordering the prosthetic aortic annulus, to the left coronary leaflet close to the left coronary artery origin (Figure 1A and B). Computed tomography (CT) showed several fistulous tracts; one connected with the ascending aorta and the other in contact with the cavity of the left ventricular outflow tract (LVOF) (Figure 1A and B).
A: Computed tomography volume rendering; the red arrow indicates the fistula with origin in the noncoronary valve and extending along the posterior part of the aortic annulus to the left coronary valve. B: Computed tomography, coronal view; the red arrow indicates the connection with the ascending aorta; the white arrow indicates connection with the left ventricular outflow tract. C: Transthoracic echocardiogram, long parasternal view, in which the regurgitation jet can be observed occupying the entire left ventricular outflow tract and leading to severe aortic insufficiency. Ao, aorta; Cx, circumflex artery; LA, left atrium; LAD, left anterior descending artery; LV, left ventricle.
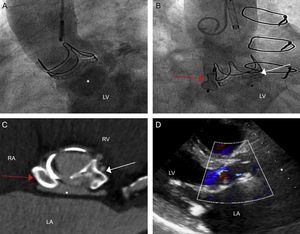
After assessing these findings and discussing the potential risks of another device-associated infection, we decided to perform percutaneous closure 3 months after the patient had completed the course of antibiotics. The patient was in agreement with this approach. Aortography showed the cavity and fistulous communication between the cavity and the LVOF, leading to severe aortic regurgitation (Figure 2A). An approach of complete sealing was considered, with closure of the entrance and exit to prevent possible rupture of the fistulous tract in the event it remained connected to the ventricle. An Amplatz-Left 5 Fr catheter with a straight hydrophilic guide was passed through the fistulous tract that led to the ascending aorta enabling access to the left ventricle. Subsequently, the guide was exchanged for a strong support guide and a PD008 device (Amplatzer Duct Occluder, St. Jude Medical) was deployed in the fistulous tract facing toward the LVOT, leaving the retaining ring in the left ventricle. Then, an Amplatzer Vascular Plug III (AVP III) 14 x 5mm device (St. Jude Medical) was deployed in the fistulous tract facing toward the ascending aorta. After deployment of both devices, a significant decrease in aortic regurgitation was observed both in the transesophageal echocardiography performed during the procedure and in the follow-up aortography (Figure 2B).
A: Aortography showing opacification of the fistula after injection of contrast (asterisk) and of left ventricle as a result of severe aortic regurgitation. B: Image after device deployment; the red arrow indicates the implanted device at the entrance (connection with the ascending aorta), the white arrow indicates the device deployed at the exit (connection with the left ventricular outflow tract). C: Computed tomography, para-axial view of the devices; the asterisk indicates the fistula, now greatly reduced in size. D: Transthoracic echocardiogram, long parasternal view, in which mild aortic insufficiency can be seen. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The postprocedural period was satisfactory and the patient was asymptomatic at 3 months after the intervention. A follow-up CT showed a decrease in the size of the cavity, which had been completely sealed by the 2 closure devices (Figure 2C). The follow-up echocardiogram showed a decrease in aortic regurgitation, which was now mild in intensity (Figure 2D), with normalization of the diameter, volume, and systolic function of the left ventricle.
Pseudoaneurysms and fistulas associated with endocarditis are infrequent entities, and patients only develop fistulas in 1.6% of cases.3 The poor prognosis of these complications is a result of the associated severe valve deterioration; in this patient, severe aortic insufficiency was present, which rapidly progressed with dilatation and ventricular dysfunction. Surgery is the first treatment option in these complications but, given the high risk of surgery often associated with these types of patients, the percutaneous option should be considered on an individual basis.
Although, recently, some cases of percutaneous closure of fistulas and pseudoaneurysms of the aortic root have been reported in this context,4,5 the use of imaging techniques such as CT as well as the use of different devices, described in this article, contributed to the successful outcome of these procedures. This case demonstrates the feasibility and safety of the percutaneous technique to correct perivalvular complications after infective endocarditis.
CONFLICTS OF INTERESTI. Cruz-González is a proctor for St. Jude Medical.