We performed a pooled analysis based on patient-level data from the TITAX-AMI and BASE-ACS trials to evaluate the outcome of titanium-nitride-oxide-coated bioactive stents vs drug-eluting stents in patients with ST-segment elevation myocardial infarction at 2-year follow-up.
MethodsThe TITAX-AMI trial compared bioactive stents with paclitaxel-eluting stents in 425 patients with acute myocardial infarction. The BASE-ACS trial compared bioactive stents with everolimus-eluting stents in 827 patients with acute coronary syndrome. The primary endpoint for the pooled analysis was major adverse cardiac events: a composite of cardiac death, recurrent myocardial infarction, or ischemia-driven target lesion revascularization at 2-year follow-up.
ResultsThe pooled analysis included 501 patients; 245 received bioactive stents, and 256 received drug-eluting stents. The pooled bioactive stent group was associated with a risk ratio of 0.85 for major adverse cardiac events (95% confidence interval, 0.53-1.35; P=.49) compared to the pooled drug-eluting stent group. Similarly, the pooled bioactive stent group was associated with a risk ratio of 0.71 for cardiac death (95% confidence interval, 0.26-1.95; P=.51), 0.44 for recurrent myocardial infarction (95% confidence interval, 0.20-0.97; P=.04), and 1.39 for ischemia-driven target lesion revascularization (95% confidence interval, 0.74-2.59; P=.30), compared to the pooled drug-eluting stent group. These results were confirmed by propensity-score adjusted analysis of the combined datasets.
ConclusionsIn patients with ST-segment elevation myocardial infarction, bioactive stents were associated with lower rates of recurrent myocardial infarction compared to drug-eluting stents at 2-year follow-up; yet, the rates of cardiac death and ischemia-driven target lesion revascularization were similar.
Keywords
First-generation drug-eluting stents (DES) effectively reduced the incidence of target lesion revascularization (TLR) by one half to two thirds at long-term follow-up.1,2 However, mounting evidence from registries and meta-analyses has questioned the long-term safety of first-generation DES, raising concerns about higher rates of late —and very late— stent thrombosis (ST).3–5 A further step forward was taken with the design of second-generation DES. In this context, the second-generation everolimus-eluting stent significantly reduced the composite endpoint of safety and efficacy, compared to the first-generation paclitaxel-eluting stent at 12-month follow-up.6,7
The safety of titanium-nitride-oxide-coated bioactive stent (BAS) has been demonstrated in several reports from observational studies in real-world populations,8,9 as well as from randomized controlled trials in patients presenting with acute coronary syndrome (ACS).10,11 In the TITAX-AMI trial, BAS was superior to paclitaxel-eluting stent for the composite endpoint of major adverse cardiac events (MACE), cardiac death, re-infarction, and definite ST in patients with acute myocardial infarction (AMI) at 5-year follow-up, with similar rates of ischemia-driven TLR.10 In the BASE-ACS trial, BAS proved noninferior to everolimus-eluting stent for the primary endpoint of MACE in patients with ACS at 12-month follow-up; nonfatal AMI was significantly lower with BAS.11 Yet, there is not much data about direct head-to-head comparison of BAS and DES in the particular setting of ST-segment elevation myocardial infarction (STEMI). Therefore, we performed a pooled analysis based on patient-level data from both the TITAX-AMI and the BASE-ACS trials to evaluate the outcome of patients with STEMI at 2-year follow-up.
METHODSPatient Selection and Study DesignThe design of both trials has previously been reported.11,12 Briefly, the TITAX-AMI trial was a prospective, single-blinded, multicenter, randomized trial conducted from December 2005 to November 2006. It included 425 patients with AMI who underwent early percutaneous coronary intervention and were randomized in a 1:1 fashion to receive either BAS (Titan-2®, Hexacath; Paris, France) or paclitaxel-eluting stent (TAXUS Liberte®, Boston Scientific; Natick, Massachusetts, United States). The primary endpoint was the first occurrence of MACE, defined as a composite of cardiac death, recurrent AMI, or ischemia-driven TLR at 12-month follow-up. The definitions of these endpoints were previously described.12 Secondary endpoints included all-cause death, a composite of cardiac death or recurrent AMI, and definite ST at 12-month follow-up. The BASE-ACS trial was a prospective, multi-center, single-blinded, randomized, noninferiority trial conducted from January 2009 to September 2010. It included 827 patients with ACS who underwent early percutaneous coronary intervention, and were randomized in a 1:1 fashion to receive either BAS or everolimus-eluting stent (Xience V®; Abbott Vascular, Santa Clara, California, United States). The primary endpoint was the first occurrence of MACE, defined as a composite of cardiac death, nonfatal AMI, or ischemia-driven TLR at 12-month follow-up. The definitions of these endpoints were also previously described.11 Secondary endpoints included all-cause death, a composite of cardiac death or nonfatal AMI, and definite ST at 12-month follow-up. We adopted the “definite” category of ST as defined by the Academic Research Consortium.13 In both trials, the operators were by necessity aware of the assigned stent, but patients and the staff involved in outcome assessment were blinded to the patient's stent group. The authors had free access to patient-level data derived from the electronic database of each trial for the current analysis. Patients presenting with STEMI who underwent early percutaneous coronary intervention from either trial were selected, and their data were combined into 2 pooled groups: those who received BAS, and those who received DES. These two pools were compared regarding their baseline clinical characteristics, angiographic and procedural data, and 2-year clinical outcome. Clinical endpoints were reported as originally defined by the authors in either trial. Analysis of the primary composite endpoint at 2-year follow-up was prespecified in both trials.
Ethical IssuesBoth studies were initiated by the investigators and conducted according to the ethical guidelines of the 1964 Declaration of Helsinki, as revised in 2002. An informed written consent was obtained from every patient after full explanation of the study protocol. The study protocol was approved by the ethics committees of the coordinating center (Satakunta Central Hospital), and the other participating hospitals. The TITAX-AMI trial was registered in Clinical Trials database14 as NCT00495664, and the BASE-ACS trial was registered as NCT00819923.
Pharmacological InterventionsFor both trials, patients already maintained on acetylsalicylic acid received no additional acetylsalicylic acid loading dose. Those not maintained on acetylsalicylic acid were pretreated with acetylsalicylic acid at a loading dose of 250mg orally or 250-500mg intravenously during percutaneous coronary intervention, and continued thereafter at a daily dose of at least 75-150mg indefinitely. Oral clopidogrel was initiated at a loading dose of at least 300mg before or immediately after the procedure and continued thereafter at a daily dose of 75mg. According to the protocol, patients in either group were prescribed oral clopidogrel for a minimum of 6 months, and thereafter for extended periods (maximum 12 months) according to the operator's discretion. During the procedure, low-molecular weight heparin (enoxaparin sodium) or unfractionated heparin was administered intravenously in the standard dosage recommended by the guidelines. Use of periprocedural glycoprotein IIb/IIIa inhibitors or bivalirudin was left up to the operator's discretion.
Statistical AnalysisDatasets from randomized studies were first combined into one single dataset for analysis without using meta-analytical statistical combination. Statistical analysis was performed using IBM SPSS (version 20.0). Continuous variables were reported as the mean ± (standard error), and dichotomous variables as counts and proportions. Pearson chi square, Kolmogorov-Smirnov, one-way analysis of variance, Kruskal-Wallis, Fisher exact, and Mann-Whitney tests were used to assess differences between the study cohorts. Although data were retrieved from randomized studies, a number of significant, or nearly significant, differences were observed between the study cohorts. Therefore, we opted to account for such differences by developing a propensity score for treatment methods. Propensity score was calculated by logistic regression, including clinical and procedural variables with a certain difference between the study groups as indicated by a P value <.2 in univariate analysis. The included studies were entered in the logistic regression model as a dichotomous covariate in order to account for any possible differences in terms of patient and procedural characteristics. Propensity score was added to the regression model as a covariate. Hosmer-Lemeshow test was used to assess the regression model fit. Receiver operating characteristic curve analysis was used to assess the predicted probability. The obtained propensity score had an area under the curve of 0.65 (95% confidence interval [95%CI], 0.60-0.69, Hosmer-Lemeshow test: P=.35). Propensity score was employed only for adjusted analysis in estimating the impact of type of stent on the post-procedural outcome. Pooled analysis of aggregate data was performed using Review Manager 5.2 software (RevMan, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). The pooled risk of adverse events was expressed as the risk ratio with 95%CI. Heterogeneity across the two trials was assessed by I2 test. When I2 was <40%, heterogeneity was considered nonsignificant. We opted to use the random-effects method because the statistical power of the I2 test is low when the number of pooled studies is small. Besides, some clinical factors point toward the presence of heterogeneity. For example, the DES group includes 2 different types of stents, and the definition of AMI as an outcome differed between the two original trials. Finally, a P value <.05 was considered statistically significant.
RESULTSA total of 501 patients with STEMI who underwent early percutaneous coronary intervention were included in the current pooled analysis; 245 patients received BAS (83 from the TITAX-AMI trial and 162 from the BASE-ACS trial) and 256 received DES (97 who received paclitaxel-eluting stents from the TITAX-AMI trial and 159 who received everolimus-eluting stents from the BASE-ACS trial). Data on the clinical outcome at 2-year follow-up were available for all patients.
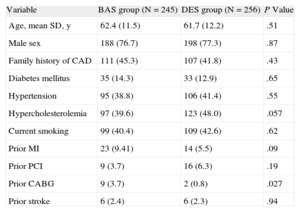
Baseline Clinical Characteristics of the Pooled CohortPatients in the BAS group were more likely to have a history of coronary bypass surgery compared to those in the DES group (3.7% vs 0.8%, respectively; P=.027), whereas those in the DES group showed a trend for more frequent hypercholesterolemia. The 2 groups were balanced for the other baseline clinical characteristics (P>.05 for all) (Table 1). Baseline clinical characteristics for the 2 subgroups in the individual trials are shown in supplementary material, Tables 1 and 2.
Baseline Clinical Characteristics of the Two Pooled Study Groups
| Variable | BAS group (N=245) | DES group (N=256) | P Value |
| Age, mean SD, y | 62.4 (11.5) | 61.7 (12.2) | .51 |
| Male sex | 188 (76.7) | 198 (77.3) | .87 |
| Family history of CAD | 111 (45.3) | 107 (41.8) | .43 |
| Diabetes mellitus | 35 (14.3) | 33 (12.9) | .65 |
| Hypertension | 95 (38.8) | 106 (41.4) | .55 |
| Hypercholesterolemia | 97 (39.6) | 123 (48.0) | .057 |
| Current smoking | 99 (40.4) | 109 (42.6) | .62 |
| Prior MI | 23 (9.41) | 14 (5.5) | .09 |
| Prior PCI | 9 (3.7) | 16 (6.3) | .19 |
| Prior CABG | 9 (3.7) | 2 (0.8) | .027 |
| Prior stroke | 6 (2.4) | 6 (2.3) | .94 |
BAS, bioactive stent; CABG, coronary artery bypass grafting; CAD, coronary artery disease; DES, drug-eluting stent; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation.
Continuous variables are presented as mean (standard deviation); dichotomous variables are presented as No. (%).
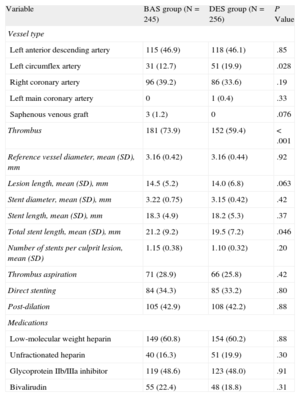
Patients in the BAS group had a longer total stent length compared to those in the DES group (21.2 [9.2] mm vs 19.5 [7.2] mm, respectively, P=.046), and had more frequent thrombus-containing lesions (73.9% vs 59.4%, respectively, P<.001). The two groups were balanced for the other angiographic and procedural data (P>.05 for all) (Table 2). Angiographic and procedural characteristics for both subgroups in the individual trials are shown in supplementary material Tables 3 and 4.
Angiographic and Procedural Data of the Two Pooled Study Groups
| Variable | BAS group (N=245) | DES group (N=256) | P Value |
| Vessel type | |||
| Left anterior descending artery | 115 (46.9) | 118 (46.1) | .85 |
| Left circumflex artery | 31 (12.7) | 51 (19.9) | .028 |
| Right coronary artery | 96 (39.2) | 86 (33.6) | .19 |
| Left main coronary artery | 0 | 1 (0.4) | .33 |
| Saphenous venous graft | 3 (1.2) | 0 | .076 |
| Thrombus | 181 (73.9) | 152 (59.4) | <.001 |
| Reference vessel diameter, mean (SD), mm | 3.16 (0.42) | 3.16 (0.44) | .92 |
| Lesion length, mean (SD), mm | 14.5 (5.2) | 14.0 (6.8) | .063 |
| Stent diameter, mean (SD), mm | 3.22 (0.75) | 3.15 (0.42) | .42 |
| Stent length, mean (SD), mm | 18.3 (4.9) | 18.2 (5.3) | .37 |
| Total stent length, mean (SD), mm | 21.2 (9.2) | 19.5 (7.2) | .046 |
| Number of stents per culprit lesion, mean (SD) | 1.15 (0.38) | 1.10 (0.32) | .20 |
| Thrombus aspiration | 71 (28.9) | 66 (25.8) | .42 |
| Direct stenting | 84 (34.3) | 85 (33.2) | .80 |
| Post-dilation | 105 (42.9) | 108 (42.2) | .88 |
| Medications | |||
| Low-molecular weight heparin | 149 (60.8) | 154 (60.2) | .88 |
| Unfractionated heparin | 40 (16.3) | 51 (19.9) | .30 |
| Glycoprotein IIb/IIIa inhibitor | 119 (48.6) | 123 (48.0) | .91 |
| Bivalirudin | 55 (22.4) | 48 (18.8) | .31 |
BAS, bioactive stent; DES, drug-eluting stent; SD, standard deviation.
Continuous variables are presented as mean (standard deviation); dichotomous variables are presented as No. (%).
The mean length of clopidogrel therapy in the BAS group compared to the DES group was 7.4 (3.1) months vs 10.4 (2.7) months (P<.001) in the TITAX-AMI trial, 8.8 (3.6) months vs 10.1 (2.9) months (P<.001) in the BASE-ACS trial, and 8.3 (3.4) months vs 10.3 (2.8) months (P<.001) in the combined series.
Propensity-Score Adjusted Clinical Outcome at 2-year Follow-upAt 2-year follow-up, the rates of MACE in the BAS vs DES study groups were 11.4% vs 13.7% (P=.50; propensity-score adjusted, odds ratio [OR]=0.78; 95%CI, 0.45-1.35), cardiac death, 2.4% vs 3.5% (P=.60; propensity-score adjusted, OR=0.65; 95%CI, 0.22-1.91), recurrent AMI 3.3% vs 7.4% (P=.032; propensity-score adjusted, OR=0.36; 95%CI, 0.15-0.88), ischemia-driven TLR 8.6% vs 6.3% (P=.39; propensity-score adjusted, OR=1.41, 95%CI, 0.70-2.83), and definite ST 0.8% vs 5.1% (P=.007, propensity-score adjusted, OR=0.31; 95%CI, 0.03-0.67), respectively.
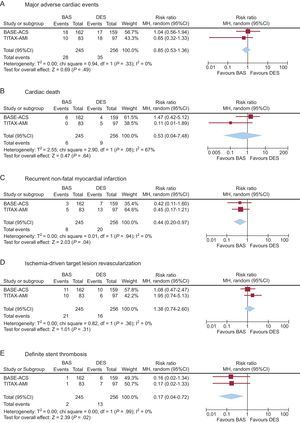
Pooled Clinical Outcome at 2-year Follow-upFigure shows forest plots of the absolute rates of clinical events in the 2 pooled groups at 2-year follow-up, with the risk ratios for the pooled group as well as the 2 individual trials. Across both trials, no evidence of heterogeneity was observed (I2=0% for all the measured events, except cardiac death, I2=67%). The pooled BAS group was associated with a risk ratio of 0.85 for MACE (95%CI, 0.53-1.36; P=.49) compared to the pooled DES group. Similarly, the pooled BAS group was associated with a risk ratio of 0.53 for cardiac death (95%CI, 0.04-7.48; P=.63); a risk ratio of 0.44 for recurrent AMI (95%CI, 0.20-0.97; P=.04); a risk ratio of 1.38 for ischemia-driven TLR (95%CI, 0.74-2.60; P=.31), and a risk ratio of 0.17 for definite ST (95%CI, 0.04-0.72; P=.02) compared to the pooled DES group (Figure).
Pooled analysis of the risk ratios of major adverse cardiac events (a composite of cardiac death, recurrent nonfatal myocardial infarction, or ischemia-driven target lesion revascularization) (A), cardiac death (B), recurrent nonfatal myocardial infarction (C), ischemia-driven target lesion revascularization (D), and definite stent thrombosis (E), associated with bioactive stents vs drug-eluting stents at 2-year follow-up. 95%CI, 95% confidence interval; BAS, bioactive stents; DES, drug-eluting stents; df, degrees of freedom; MH, Mantel-Haenszel.
The current pooled analysis of the TITAX-AMI and BASE-ACS trials at 2-year follow-up demonstrated that in patients with STEMI who underwent early percutaneous coronary intervention, implantation of BAS, compared to DES, was associated with significantly lower rates of recurrent AMI (P=.04), and definite ST (P=.02). The rates of cardiac death, ischemia-driven TLR, and the composite of MACE were statistically similar (P>.05 for all). To the best of the authors’ knowledge, the current report is the first pooled analysis of randomized trials reported to date, presenting clinical outcome of BAS vs DES in the setting of STEMI.
Efficacy and Safety EndpointsIn the current pooled analysis, BAS implantation in patients with STEMI was associated with a slight (15%) reduction of the relative risk of the composite outcome of efficacy and safety at 2-year follow-up, compared to a pooled group of DES (paclitaxel-eluting stents and everolimus-eluting stents) (P=.49). Not surprisingly, there was a 39% increase in the relative risk of the device-specific efficacy endpoint of ischemia-driven TLR (P=.30). The fact that no routine angiographic follow-up was performed may have influenced the relative rates of TLR between the 2 stent groups. It is well-known that angiographic follow-up increases the absolute differences between stents with respect to TLR beyond that which would otherwise be seen with clinical follow-up alone. Nevertheless, adopting solo clinical follow-up, the results would reflect real-world practice, avoiding repeat interventions for clinically “silent” angiographic lesions. The increase in the relative risk of TLR associated with BAS was balanced by 56% relative risk reduction of recurrent AMI (P=.04), and 29% relative risk reduction of cardiac death (P=.51) (safety endpoints). The reduction of risk of recurrent AMI was most likely driven by 83% relative risk reduction of definite ST associated with BAS vs DES at 2 years (P=.02).
Current Literature PerspectiveIn a 12-month post-hoc analysis of the BASE-ACS trial based on the type of ACS, the relative risk ratio associated with BAS vs everolimus-eluting stent in patients with STEMI was 1.06 for MACE, 1.1 for ischemia-driven TLR, 0.27 for recurrent AMI, 1.95 for cardiac death, and 0.16 for definite ST.15 In that post-hoc analysis, the 12-month rates of recurrent AMI and cardiac death associated with everolimus-eluting stents were 4.4% and 1.9%, respectively; definite ST was 3.8%. The composite rate of MACE was 8.8%.15 Yet, the event rates associated with DES in the current pooled analysis are relatively higher than those reported in other randomized trials. In a 2-year report of pooled data from the SPIRIT and the COMPARE trials, the event rates associated with everolimus-eluting stents in patients with ACS were as follows: MACE, 8.7%; cardiac death, 1.4%; nonfatal AMI, 4%; ischemia-driven TLR, 4.7%, and definite ST, 0.4%.16 The enrollment of patients with the full spectrum of ACS, vs those with STEMI in the current study, and the fact that the current pooled analysis included the outcome of both paclitaxel- and everolimus-eluting stents, might explain the differences between the 2-year outcomes of the 2 pooled analyses. Evidence from the SPIRIT trial program and the COMPARE trial underscores that the outcomes of paclitaxel- and everolimus-eluting stents are not comparable: everolimus-eluting stents demonstrated superior efficacy (reduction of in-stent and in-segment late loss) and safety (reduction of AMI and ST).7,17–19 In the EXAMINATION trial comparing everolimus-eluting stents vs bare-metal stents in patients presenting with STEMI, the 12-month rates of recurrent target-vessel-related AMI and cardiac death associated with everolimus-eluting stents were 1.1% and 3.2%, respectively; definite ST was 0.5%.20 The lower event rates can be explained by the shorter follow-up period, compared to the current pooled analysis. Additionally, the trial adopted the World Health Organization extended definition of AMI, which is based on elevation of total creatine kinase>2 times the upper reference limit (URL), creatine kinase MB fraction>3 times URL, or troponin>3 times URL, in hierarchical order.21 This is less sensitive than the definition used in either the BASE-ACS trial (creatine kinase MB fraction or troponin >2 times URL) or the TITAX-AMI trial (troponin >99th percentile of URL).11,12 Moreover, the lower incidence of ST might be attributed to more frequent thrombectomy in the EXAMINATION trial (66%).20 Similarly, the XAMI trial compared everolimus-eluting stents vs sirolimus-eluting stents in the setting of primary percutaneous coronary intervention for STEMI.22 The rates of recurrent AMI and cardiac death associated with everolimus-eluting stents at 12 months were 0.5% and 1.5%, respectively; the rate of definite or probable ST was 1.2%.22 The lower rates of recurrent AMI might again be seen in light of the shorter period of follow-up. Furthermore, the definition used in the trial was based on a total creatine kinase >3 times URL. The lower rate of ST observed might possibly be attributed to the higher frequency of thrombus aspiration (61.9%) and glycoprotein IIb/IIIa inhibitor administration (74.5%) in the XAMI trial.22 Finally, the PASSION trial compared paclitaxel-eluting stents vs bare-metal stents in primary percutaneous coronary intervention for STEMI. The 2-year rates of recurrent AMI, cardiac death, and definite ST associated with paclitaxel-eluting stents were 3.1%, 5.6%, and 2.1%, respectively.23 Again, the definition of AMI was different from that used in the TITAX-AMI trial; recurrent AMI was defined based on either the appearance of pathological Q waves, or total creatine kinase >2 times URL with an elevation of creatine kinase MB fraction.23
Primary Angioplasty in ST-segment Elevation Myocardial InfarctionRandomized trials comparing the efficacy and safety of DES vs bare-metal stents in the setting of STEMI consistently demonstrated that DES reduced the need for repeat revascularization, with no statistically significant difference in the incidence of ST.24 The largest trial so far, HORIZONS-AMI (N=3006), showed superiority of paclitaxel-eluting stents for the primary efficacy endpoint of ischemia-driven TLR (P=.002), with noninferiority for the composite safety endpoint (P=.92, P noninferiority=. 01), compared to bare-metal stents.25 In a pooled analysis of 389 diabetic patients from 7 randomized controlled trials comparing DES vs bare-metal stents in STEMI, the rate of TLR was significantly lower in those treated with DES (hazard ratio=0.44; P=.02), with no significant difference in the combined safety endpoint of death or AMI (hazard ratio=0.64; P=.12).26
Limitations of the StudyAlthough the current pooled analysis was performed in order to increase the statistical power of the comparison groups to detect any difference in clinical outcome, the rate of occurrence of the individual adverse events may limit the capacity to detect a difference between the 2 treatment strategies. Therefore, the current pooled analysis may not be adequately powered to address the individual components of safety and efficacy; and therefore, the results of the current pooled analysis should be taken as hypothesis-generating rather than conclusive. In specific, the differences in AMI and ST in favor of BAS vs everolimus-eluting stents should be interpreted with caution. Moreover, only 2 randomized trials were available for pooled analysis. The fact that the 2 pooled trials employed 2 different DES with discrete drug and polymer characteristics, dissimilar biological behavior, and diverse clinical outcomes might constitute another important limitation. Furthermore, although the definitions used for the individual adverse events were fairly uniform between the 2 trials, the definition of recurrent AMI was slightly different: in the TITAX-AMI trial it included all recurrent AMI, whereas in the BASE-ACS trial it included only target-vessel related AMI. Missing data on acute procedural outcome such as TIMI (Thrombolysis In Myocardial Infarction) flow grade and post-procedural ST segment resolution, and similarly, missing data about the timing of events such as AMI and ST, are other study limitations. Limitations of aggregated data analysis for summarizing a few studies include inappropriate accounting for heterogeneity, selective reporting, and publication bias. Finally, the fact that TLR was ischemia-driven may have underestimated the actual rates of in-stent restenosis; however, it would avoid unnecessary re-interventions in borderline restenotic lesions due to the “oculostenotic reflex” and undue patient anxiety.
CONCLUSIONSThe current pooled analysis of the TITAX-AMI and BASE-ACS trials at 2-year follow-up demonstrated that in patients with STEMI who underwent early percutaneous coronary intervention, implantation of BAS was associated with lower rates of recurrent AMI and definite ST, compared to DES; yet, the rates of cardiac death and ischemia-driven TLR were similar.
CONFLICTS OF INTERESTNone declared.