The aim of this study was to analyze the prevalence, risk factors, and short- and long-term prognosis of patients with acute coronary syndrome and normal renal function who developed percutaneous coronary intervention-associated nephropathy.
MethodsThis was an observational, retrospective, single-center study with a prospective follow-up of 470 consecutive patients hospitalized for acute coronary syndrome (not in cardiogenic shock) who underwent percutaneous coronary intervention, with no preexisting renal failure (admission creatinine ≤ 1.3mg/dL). Percutaneous coronary intervention-associated was defined as an increase in baseline creatinine ≥ 0.5 mg/dL or ≥ 25% baseline. The mean follow-up was 26.7 (14) months.
ResultsOf the 470 patients, 30 (6.4%) developed percutaneous coronary intervention-associated nepfhropathy. The independent predictors for acute renal failure were admission hemoglobin level (odds ratio = 0.71) and maximum troponin I level prior to the procedure (odds ratio = 1.02). During the long-term follow-up, the patients whose renal function deteriorated had a higher incidence of total mortality (5 [16.7%] vs 27 [6.1%]; P = .027). In the Cox regression analysis, percutaneous coronary intervention-associated nepfhropathy was not an independent predictor for total mortality, but could be a predictor for cardiac mortality (hazard ratio=5.4; 95% confidence interval 1.35-21.3; P = .017).
ConclusionsPercutaneous coronary intervention-associated nephropathy in patients with acute coronary syndrome and normal preexisting renal function is not uncommon and influences long-term survival.
Keywords
Increased plasma creatinine levels after percutaneous coronary intervention (PCI) are not uncommon and have been associated with adverse prognoses.1 The development of PCI-associated nephropathy is associated with a substantial increase in morbidity and mortality, prolonged hospital stays, and higher hospital costs.2–4 The causes of acute renal failure after a percutaneous coronary revascularization procedure vary greatly and include contrast-induced nephrotoxicity, hemodynamic alterations, drug-induced toxicity and atheroembolism. The greatest risk factor for PCI-associated nephropathy is the degree of preexisting renal insufficiency; therefore, nephroprotective measures usually center on this patient subgroup.3,5,6 Nonetheless, patients with acute coronary syndrome (ACS) treated with PCI are also a population with a higher risk for subsequent nephropathy.4,7 Few studies have specifically evaluated the long-term prognosis and prevalence of PCI-associated nephropathy in patients with ACS and normal renal function. While the best treatment for this serious complication is prevention, the current guidelines of the European Society of Cardiology for the management of patients with ACS without persistent ST-segment elevation only recommend nephroprotective measures for patients with ACS and chronic renal failure.8 Therefore, studies including patients with normal renal function could identify a subgroup of patients who would also benefit from these preventative measures.
The aim of this study was to analyze the prevalence, risk factors, and in-hospital and long-term prognoses of patients with ACS without preexisting renal insufficiency who experienced a decline in renal function after a percutaneous coronary revascularization procedure.
METHODSFrom 2007-2011, a total of 602 consecutive patients underwent PCI due to ACS either with or without ST-segment elevation. Excluded from the analysis were those patients with renal insufficiency detected at admission (admission creatinine level>1.3mg/dL, n=126) and those with cardiogenic shock (n=6). The study group included 470 patients (mean age 65 [12]; women, 89 [18.9%]) with ACS and without preexisting renal insufficiency or cardiogenic shock who underwent percutaneous revascularization.
This was an observational, retrospective, single-center study with prospective collection of the variables. Several clinical and epidemiological variables were recorded in the department database, including age, sex, cardiovascular risk factors, other comorbidities, and previous treatment. On admission, a full work-up with complete blood count and biochemical profile was done, with myocardial necrosis marker measurements (6h and 12h) and complete analysis at 8 am on the day after admission. After the PCI, and as part of the standard protocol at our hospital, new myocardial necrosis marker curves were calculated 6h and 12h later. Renal function was also assessed on the following day. The quantity and the timing of further bloodwork and analyses were determined according to the criteria of the treating physician. Creatinine levels were recorded at admission and during hospitalization. Creatinine clearance was estimated with the simplified Modification of Diet in Renal Disease formula.9,10
Catheterization and TreatmentThe PCI was performed in accordance with the standard technique, usually using radial access (394 [83.8%]). In all cases, the contrast media used were iohexol (Omnipaque 350® and Omnipaque 300®) and iodixanol (Visipaque 320®). We recorded the number of main vessels affected in the coronary arteriography, type of access, contrast dose, fluoroscopy time, number of stents used, and their type. We also took note of the antithrombotic treatment used, hemorrhagic complications according to the route of access, angiographic success, and the presence of complications during the procedure. The decision to establish a hydration regime through catheterization, the type and dosage of fluid therapy, and the need for renal replacement therapy was left to the discretion of the treating physician.
Follow-up and ObjectivesAt our hospital, all the patients who underwent PCI were prospectively followed up for a minimum of 12 months (mean follow-up: 26.7 [14.0] months). The follow-up data were compiled from a review of the electronic case files at our hospital and standardized telephone interviews were systematically performed after 1 month, 1 year, and at the end of the follow-up period. The objectives analyzed included mortality and in-hospital complications, cardiovascular events, and long-term cardiac mortality and total mortality.
DefinitionsPercutaneous coronary intervention-associated nephropathy was defined as an increase of ≥ 25% or ≥ 0.5mg/dL in serum creatinine levels in the 72h following the procedure.6,11,12 Acute myocardial infarction was defined as troponin I elevation due to acute myocardial ischemia, and post-PCI infarction was defined according to the criteria of the latest universal classification of myocardial infarction (troponin I elevation > 1ng/mL in patients with normal baseline levels or an increase of >20% in patients with elevated troponin in a situation of stability or decrease, associated with clinical data, electrocardiographic data, images of ischemia or angiographic findings compatible with some type of complication).13 Left ventricular ejection fraction (LVEF) was estimated by echocardiogram. Cardiogenic shock was defined as systolic blood pressure<90mmHg for ≥1h accompanied by tissue hypoperfusion requiring inotropic support and/or implantation of an intra-aortic balloon pump. Cardiac mortality was that resulting from ACS, heart failure, or ventricular arrhythmia. Angiographic success was defined as the presence of a Thrombolysis In Myocardial Infarction flow ≥ 2 in the absence of residual stenosis > 50%. Hemorrhagic complications related to the vascular access were classified using the Bleeding Academic Research Consortium scale.14 Revascularization of the target lesion was defined as the need for a new revascularization procedure (either percutaneous or surgical) of the coronary segment with a stent, done in the presence of angiographic restenosis (stenosis > 50%) and symptoms or signs of myocardial ischemia. Revascularization of the target vessel was defined as new percutaneous or surgical revascularization of the previously-treated vessel in the presence of severe stenosis (> 70%) and symptoms or signs of myocardial ischemia.
Statistical AnalysisFor the analysis of the data, we used the SPSS version 20 statistical package. The quantitative variables are reported as mean (standard deviation). The categorical variables are described as absolute value and percentage. The differences in the results of a quantitative variable between the 2 patient groups were analyzed with Student's t test, while the differences between categorical variables were analyzed with Pearson's chi-square or Fisher's exact tests, as required. A stepwise backward logistic regression analysis was used to determine the independent predictive factors for PCI-associated nephropathy. The variables included in this analysis correlated significantly with the appearance of kidney dysfunction in the univariate model and those that were considered to have clinical relevance, including: hemoglobin at admission, maximum troponin I prior to PCI, maximum creatine kinase prior to PCI, glomerular filtration rate, LVEF, contrast volume, and C-reactive protein at admission. All these variables were collected before the appearance of PCI-associated nephropathy. The results are expressed as odds ratio (OR) and 95% confidence interval (95%CI). Total death-free survival and cardiac death-free survival in the groups with and without PCI-associated nephropathy were compared with the Kaplan-Meier analysis (log rank test). A backward stepwise multivariate Cox regression analysis was done to determine the independent predictors for total and cardiac mortality. The variables included in these analyses are described as the main predictors for mortality in the context of ACS8: age, LVEF (continuous variable), hemoglobin at admission (continuous variable), diabetes mellitus, and PCI-associated nephropathy. The results are expressed as hazard ratios (HR) and their 95%CI. A P < .05 was considered statistically significant.
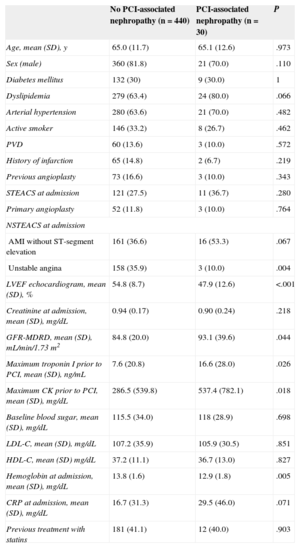
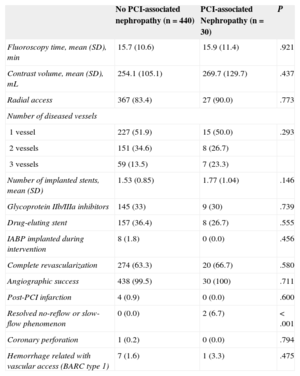
RESULTSBaseline Characteristics and Prevalence of Percutaneous Coronary Intervention-associated NephropathyOf the 470 patients analyzed, 30 (6.4%; 95%CI, 4.3%-8.9%) developed PCI-associated nephropathy, despite normal plasma creatinine values at admission; 2 patients (0.4%) required dialysis. The baseline characteristics, angiographic data and data from the interventional procedure of the patients who developed PCI-associated nephropathy vs those who did not are shown in Tables 1 and 2.
Clinical and Laboratory Data of Patients According to Whether They Developed Percutaneous Coronary Intervention-associated Nephropathy
| No PCI-associated nephropathy (n=440) | PCI-associated nephropathy (n = 30) | P | |
|---|---|---|---|
| Age, mean (SD), y | 65.0 (11.7) | 65.1 (12.6) | .973 |
| Sex (male) | 360 (81.8) | 21 (70.0) | .110 |
| Diabetes mellitus | 132 (30) | 9 (30.0) | 1 |
| Dyslipidemia | 279 (63.4) | 24 (80.0) | .066 |
| Arterial hypertension | 280 (63.6) | 21 (70.0) | .482 |
| Active smoker | 146 (33.2) | 8 (26.7) | .462 |
| PVD | 60 (13.6) | 3 (10.0) | .572 |
| History of infarction | 65 (14.8) | 2 (6.7) | .219 |
| Previous angioplasty | 73 (16.6) | 3 (10.0) | .343 |
| STEACS at admission | 121 (27.5) | 11 (36.7) | .280 |
| Primary angioplasty | 52 (11.8) | 3 (10.0) | .764 |
| NSTEACS at admission | |||
| AMI without ST-segment elevation | 161 (36.6) | 16 (53.3) | .067 |
| Unstable angina | 158 (35.9) | 3 (10.0) | .004 |
| LVEF echocardiogram, mean (SD), % | 54.8 (8.7) | 47.9 (12.6) | <.001 |
| Creatinine at admission, mean (SD), mg/dL | 0.94 (0.17) | 0.90 (0.24) | .218 |
| GFR-MDRD, mean (SD), mL/min/1.73 m2 | 84.8 (20.0) | 93.1 (39.6) | .044 |
| Maximum troponin I prior to PCI, mean (SD), ng/mL | 7.6 (20.8) | 16.6 (28.0) | .026 |
| Maximum CK prior to PCI, mean (SD), mg/dL | 286.5 (539.8) | 537.4 (782.1) | .018 |
| Baseline blood sugar, mean (SD), mg/dL | 115.5 (34.0) | 118 (28.9) | .698 |
| LDL-C, mean (SD), mg/dL | 107.2 (35.9) | 105.9 (30.5) | .851 |
| HDL-C, mean (SD) mg/dL | 37.2 (11.1) | 36.7 (13.0) | .827 |
| Hemoglobin at admission, mean (SD), mg/dL | 13.8 (1.6) | 12.9 (1.8) | .005 |
| CRP at admission, mean (SD), mg/dL | 16.7 (31.3) | 29.5 (46.0) | .071 |
| Previous treatment with statins | 181 (41.1) | 12 (40.0) | .903 |
AMI, acute myocardial infarction; CK, creatine kinase; CRP, C-reactive protein; GFR-MDRD, glomerular filtration rate-Modification of Diet in Renal Disease; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEACS, ST-segment elevation acute coronary syndromes; NSTEACS, non—ST-segment elevation acute coronary syndromes; SD, standard deviation.
Data are expressed as mean (standard deviation) or No. (%).
Angiographic Data and Interventional Procedures Data According to the Development of Percutaneous Coronary Intervention-associated Nephropathy
| No PCI-associated nephropathy (n=440) | PCI-associated Nephropathy (n=30) | P | |
|---|---|---|---|
| Fluoroscopy time, mean (SD), min | 15.7 (10.6) | 15.9 (11.4) | .921 |
| Contrast volume, mean (SD), mL | 254.1 (105.1) | 269.7 (129.7) | .437 |
| Radial access | 367 (83.4) | 27 (90.0) | .773 |
| Number of diseased vessels | |||
| 1 vessel | 227 (51.9) | 15 (50.0) | .293 |
| 2 vessels | 151 (34.6) | 8 (26.7) | |
| 3 vessels | 59 (13.5) | 7 (23.3) | |
| Number of implanted stents, mean (SD) | 1.53 (0.85) | 1.77 (1.04) | .146 |
| Glycoprotein IIb/IIIa inhibitors | 145 (33) | 9 (30) | .739 |
| Drug-eluting stent | 157 (36.4) | 8 (26.7) | .555 |
| IABP implanted during intervention | 8 (1.8) | 0 (0.0) | .456 |
| Complete revascularization | 274 (63.3) | 20 (66.7) | .580 |
| Angiographic success | 438 (99.5) | 30 (100) | .711 |
| Post-PCI infarction | 4 (0.9) | 0 (0.0) | .600 |
| Resolved no-reflow or slow-flow phenomenon | 0 (0.0) | 2 (6.7) | <.001 |
| Coronary perforation | 1 (0.2) | 0 (0.0) | .794 |
| Hemorrhage related with vascular access (BARC type 1) | 7 (1.6) | 1 (3.3) | .475 |
BARC, Bleeding Academic Research Consortium; IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; SD, standard deviation.
Data are expressed as mean (standard deviation) or No. (%).
The group of patients who developed PCI-associated nephropathy showed lower LVEF and hemoglobin concentrations prior to PCI, greater elevation in myocardial necrosis markers (troponin I and creatine kinase), a lower prevalence of unstable angina, a tendency toward higher C-reactive protein levels, and a greater prevalence of dyslipidemia and infarction without ST-segment elevation. In the logistic regression analysis, the independent predictive factors for PCI-associated nephropathy were admission hemoglobin concentration (OR=0.706; 95%CI, 0.545-0.914; P=.008) and a maximum troponin I value prior to PCI (OR=1.016; 95%CI, 1.002-1.031; P=.025).
Prognosis of Percutaneous Coronary Intervention-associated NephropathyIn-hospital Clinical CourseDuring the hospital follow-up, patients with PCI-associated nephropathy had a higher proportion of coronary slow-flow or resolved no-reflow phenomena (6.7% vs 0%; P<.001) and greater in-hospital mortality (6.7% vs 0.5%; P<.001). There was not, however, a greater number of hemorrhagic complications related to the vascular access nor was there a greater presence of post-PCI infarction. All the hemorrhagic complications related to the vascular access were Bleeding Academic Research Consortium type 1 (mild hemorrhages not requiring additional studies or treatment and not prolonging hospital stay).
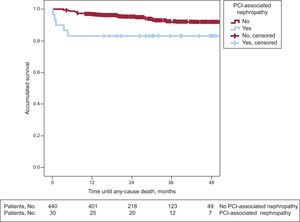
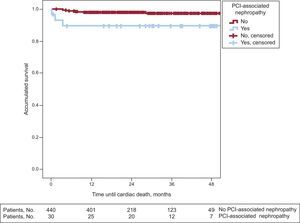
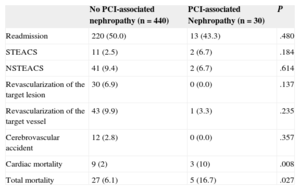
Long-term Follow-upIn the long-term follow-up, patients with PCI-associated nephropathy showed a higher incidence of cardiac mortality (3 [10%] vs 9 [2%]; P=.008) and total mortality (5 [16.7%] vs 7 [6.1%]; P=.027) (Table 3). The patients whose renal function deteriorated after PCI had shorter survival in the long-term follow-up (43 [3.4] months vs 51.4 [0.6] months; log rank test, P=.024). Figures 1 and 2 show the survival curves for total and cardiac mortality stratified according to the development of PCI-associated nephropathy.
Long-term Events in Patients With and Without Percutaneous Coronary Intervention-associated Nephropathy
| No PCI-associated nephropathy (n=440) | PCI-associated Nephropathy (n=30) | P | |
|---|---|---|---|
| Readmission | 220 (50.0) | 13 (43.3) | .480 |
| STEACS | 11 (2.5) | 2 (6.7) | .184 |
| NSTEACS | 41 (9.4) | 2 (6.7) | .614 |
| Revascularization of the target lesion | 30 (6.9) | 0 (0.0) | .137 |
| Revascularization of the target vessel | 43 (9.9) | 1 (3.3) | .235 |
| Cerebrovascular accident | 12 (2.8) | 0 (0.0) | .357 |
| Cardiac mortality | 9 (2) | 3 (10) | .008 |
| Total mortality | 27 (6.1) | 5 (16.7) | .027 |
PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome; NSTEACS, non—ST-segment elevation acute coronary syndrome.
Data are expressed as No. (%).
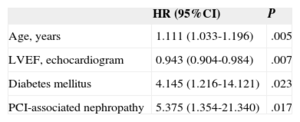
In the Cox multivariate regression analysis, the independent predictors for total mortality were age (HR=1.079; 95%CI, 1.032-1.127; P=.001), LVEF (HR=0.962; 95%CI, 0.936-0.988; P=.005), and hemoglobin concentration prior to the procedure (HR=0.659; 95%CI, 0.531-0.819; P<.001). The independent predictors for cardiac mortality were age, LVEF, diabetes mellitus, and PCI-associated nephropathy (HR=5.4; 95%CI, 1.35-21.30; P=.017) (Table 4).
Cox Multivariate Regression Analysis: Independent Predictors for Cardiac Mortality
| HR (95%CI) | P | |
|---|---|---|
| Age, years | 1.111 (1.033-1.196) | .005 |
| LVEF, echocardiogram | 0.943 (0.904-0.984) | .007 |
| Diabetes mellitus | 4.145 (1.216-14.121) | .023 |
| PCI-associated nephropathy | 5.375 (1.354-21.340) | .017 |
95%CI, 95% confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Contrast-induced nephropathy is a common complication in patients who have undergone invasive procedures and has been associated with increased morbidity and mortality.1–4 The incidence of contrast-induced nephropathy varies in published studies. This variability is a result of the differences in the presence of risk factors, the quantity and type of contrast used, and the definition of contrast-induced nephropathy adopted by each study. Although the risk of contrast-induced nephropathy in the general population is very low,12 its incidence can be considerably higher in certain risk subgroups. The identification of patients at risk of developing PCI-associated nephropathy could be of immense value, since prophylactic treatment could be administered to high-risk populations.
For better patient stratification, efforts have been made to develop tools or risk scales that could identify patients most likely to develop PCI-associated nephropathy.6,15 In this context, baseline renal function is the most powerful predictor for contrast-induced nephropathy after PCI.3–6 In fact, current guidelines only recommend the use of prophylactic treatment for patients with altered renal function,8 although this approach has limited sensitivity and specificity.
Few studies have evaluated PCI-associated nephropathy in the subgroup of patients with normal renal function. We have found only 1 study that evaluated the incidence and the results of PCI-associated nephropathy nephropathy in patients with normal renal function, which was written by Roy et al16 and analyzed only diabetic patients. Rihal et al4 identified acute myocardial infarction as an independent predictor of acute renal failure after cardiac catheterization. Therefore, the population analyzed in our study (patients with ACS and normal renal function) is one that is not often evaluated in the literature.
This present study demonstrates that PCI-associated nephropathy in patients with ACS and normal renal function is not uncommon (6.4%; 95%CI, 4.3%-8.9%). In this patient subgroup, the independent predictors for PCI-associated nephropathy were hemoglobin concentrations at admission and troponin levels before PCI. Patients with ACS with low hemoglobin concentrations at admission and elevated myocardial necrosis markers (fundamentally troponin) should be monitored more closely, and systematic nephroprotective strategies should be considered. The limited number of diabetic patients (141), together with the exclusion of those with diabetic nephropathy, could explain why we did not detect a greater risk of PCI-associated nephropathy in the diabetic patients in our sample.
Currently, no treatment has been established as the best option for the prevention of PCI-associated nephropathy. Nonetheless, there are a series of recommendations for prophylaxis with demonstrated utility in published studies, such as withdrawal of nephrotoxic drugs,11 use of lower doses of contrast material, low osmolarity or iso-osmolar contrast media,17,18 avoidance of repeated procedures for 48–72h, avoidance of situations of hypovolemia, and the use of different hydration protocols,19,20 either in combination with N-acetylcysteine or alone.21,22 Recio-Mayoral et al23 have reported that a rapid-hydration regime with sodium bicarbonate and N-acetylcysteine was effective in the prevention of contrast-induced nephropathy in patients who underwent urgent catheterization.
Prognosis of Percutaneous Coronary Intervention-associated NephropathyHospital mortality was higher in patients who developed PCI-associated nephropathy (6.7% vs 0.5%; P<.001). Previous studies have published hospital mortality rates of 13.9% in patients with a decline in renal function after urgent cardiac catheterization, compared with 0.6% of the population that did not develop renal insufficiency.7
Patients who survive an episode of acute renal failure after a percutaneous revascularization procedure may continue to be at risk for further events in the long-term. In our study, with a mean follow-up of 26.7 (14) months, total and cardiac mortality rates were strikingly higher in the group that developed PCI-associated nephropathy. In addition, these curves showed that the reduction in survival essentially took place in the first 12 months after the decline in renal function. It is possible that patients with normal renal function who develop PCI-associated nephropathy have a poorer prognosis in the first 12 months and that afterward, as the creatinine levels in these patients usually normalize during follow-up, their risk becomes similar to that of the rest of the population.
Therefore, our data show evidence of the negative prognostic impact of PCI-associated nephropathy, regardless of baseline serum creatinine levels. Although PCI-associated nephropathy can be a marker for hemodynamic deterioration and other comorbidities, which at the same time are important in the prognosis of these patients, it was shown to be a possible independent predictor for cardiac mortality after adjustment for these variables. For this reason, the renal function of all ACS patients scheduled to undergo PCI should be monitored, including those patients with normal renal function at admission. It is likely that PCI-associated nephropathy in our hemodynamically-stable patient population (no cardiogenic shock) may be a sign of greater myocardial damage or more aggressive atherosclerosis, which would fundamentally affect cardiac mortality. In our study, cardiac mortality represented only 37.5% of total mortality, which could explain why we did not detect PCI-associated nephropathy as an independent predictor for total mortality.
Strengths and LimitationsAlthough the demographic, clinical and angiographic data were collected prospectively, ours is a retrospective analysis, with the limitations inherent to this type of study. The sample size may have limited the power of our study to detect a significant association between the decline in renal function after PCI and other risk factors for PCI-associated nephropathy, such as diabetes mellitus or the use of contrast volume. We did not collect data for variables with a strong impact on mortality, such as heart rate, blood pressure, and Killip class. If these variables had been recorded, the predictive models and, therefore, the prognostic value of PCI-associated nephropathy might have been different. After the first 24h, analyses were ordered at the discretion of the treating physician, which may have led to a failure to identify some patients with PCI-associated nephropathy. Given that patients with ACS are hospitalized for several days and undergo several batteries of tests, that acute renal injury after contrast injection usually takes place within the first 3 days after the procedure, and that elevated serum creatinine levels are seen within the first 24h in 80% of cases,11 we believe that the possible number of unidentified patients was probably quite minimal. The study design does not allow us to determine the relative importance of atheroembolism or hemodynamic alterations with regard to the administration of contrast material in the development of renal dysfunction. Because of the low number of cardiac deaths (12 patients), the results of the multivariate analysis related to this event should be interpreted with caution, although this finding may be an important generator of hypotheses for studies with larger samples.
One of the strong points of the study is that the results are based on a detailed consecutive register of patients in our healthcare region who had been hospitalized due to ACS. From the database, we obtained a large quantity of clinical, analytical, and angiographic information during their hospital stay, and several variables related to their clinical course were recorded over a very long period of time.
CONCLUSIONSPercutaneous coronary intervention-associated nephropathy in patients with ACS and normal renal function is not an uncommon complication. In these patients, hemoglobin concentrations at admission and troponin values prior to the interventionism were independent predictors for PCI-associated nephropathy. This complication is a marker for poor in-hospital and long-term prognosis. For this reason, renal function should be monitored in all patients with ACS scheduled to undergo PCI, and nephroprotective strategies should be considered even for patients with normal renal function at admission, especially in the subgroup of patients with anemia and elevated troponin.
CONFLICTS OF INTERESTNone declared.