The scientific evidence for using beta-blockers after acute coronary syndrome stems from studies conducted in the days before coronary revascularization and in patients with ventricular dysfunction. The aim of this study was to analyze the current long-term prognostic benefit of beta-blockers in patients with acute coronary syndrome and preserved left ventricular ejection fraction.
MethodsWe conducted a retrospective cohort study of 3236 patients with acute coronary syndrome and left ventricular ejection fraction ≥ 50%. We performed a propensity-matched analysis to draw up two groups of 555 patients paired according to whether or not they had been treated with beta-blockers. The prognostic value of beta-blockers to predict mortality during follow-up was analyzed using Cox regression.
ResultsDuring the follow-up (median, 5.2 years), 506 patients (15.6%) died. Patients treated with beta-blockers (n=2277 [70.4%]) had a lower mortality rate (11.6% vs 25.2%; P<.001). After propensity score matching, we found that mortality during follow-up was still lower in the beta-blocker group (14.4% vs 18.9%; P=.020). Therefore, this treatment was an independent protective factor after adjusting for confounding variables in the multivariate Cox regression analysis (hazard ratio=0.64; 95% confidence interval, 0.48-0.87; P=.004).
ConclusionsBeta-blocker treatment in patients with acute coronary syndrome and preserved left ventricular ejection fraction is associated with lower long-term mortality.
Keywords
The current American College of Cardiology and European Society of Cardiology guidelines recommend starting beta-blockers in all patients with acute coronary syndrome (ACS) within a few hours of onset and continuing indefinitely at discharge.1–4 These recommendations are particularly strong for patients with reduced left ventricular ejection fraction (LVEF).
However, clinical practice guidelines that recommend the use of beta-blockers after ACS are based on scientific evidence from studies conducted in the pre-percutaneous revascularization era.5–13 Since the introduction of percutaneous coronary intervention (PCI), the protective role of beta-blockers is less clear, and appears to apply more to high-risk patients14 such as those with multivessel disease,15 previous myocardial infarction16, and reduced LVEF.17 There is little evidence supporting the benefit of beta-blockers after an ACS in patients with preserved LVEF function in the current era.
To investigate this issue, we conducted a study based on propensity score matching to assess the prognostic benefit of beta-blockers on mortality in a contemporary cohort of patients with ACS and preserved left ventricular systolic function.
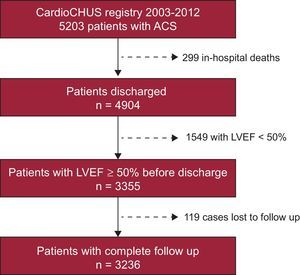
METHODSStudy PopulationWe based our retrospective cohort study on all patients entered in the CardioCHUS registry who were consecutively admitted with a diagnosis of ACS to the Cardiology Department of Hospital Clínico de Santiago de Compostela (consisting of the coronary unit, intermediate care, and wards) between December 2003 and September 2012 (n=5203). We selected patients surviving the hospital stay (n=4904) and then filtered for those who had an LVEF ≥ 50 at discharge, calculated using Simpson's rule (n=3355) (Figure 1). Follow-up data were available for 96.5% of these patients, and therefore the study cohort consisted of 3236 patients. Cardiologists from our department prospectively collected patients’ demographic, clinical, and angiographic data as well as details on treatment and follow-up. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Study Objective and Follow-upWe divided patients into two groups, according to whether they were receiving beta-blocker treatment at discharge (n=2277 [70.4%]) or not (n=959 [19.6%]). The clinical cardiologist in charge of each patient decided whether to use beta-blocker treatment. The primary study endpoint was the effect of beta-blocker treatment on overall mortality during follow-up (median, 5.2 years [interquartile range, 2.0-7.2 years]). After discharge, patients were followed up at an ischemic heart disease clinic and by their general physicians. Our structured follow-up was based on each patient's unique electronic health record (IANUS program, Galicia autonomous community), reviewing all medical contacts and hospital notes. We followed up by telephone in some cases.
Statistical analysisQuantitative variables were expressed as mean (standard deviation) and we used Student t test for between-group comparisons. Categorical variables were expressed as a percentage and we compared them using chi-square. Since this study had a nonrandomized design and multiple factors affect the decision to start beta-blocker treatment, we performed a propensity-matched analysis to reduce the bias from studying treatment effect in an observational context. We used the propensity score to assess the probability of a patient receiving beta-blocker treatment according to his or her baseline characteristics. We then matched the propensity scores, a statistical technique that equates group characteristics using defined variables to analyze the effect of a single variable. In our case, this variable was nonrandomized beta-blocker treatment at discharge. We applied a greedy 1:1 matching algorithm without replacement and defined optimal matching as a standard deviation of 0.2. Binary logistic regression was used for the analysis, where the dependent variable was beta-blocker treatment (yes/no) and explanatory variables were age (continuous variable), female sex, diabetes mellitus, smoker, peripheral artery disease, chronic obstructive pulmonary disease/asthma, previous myocardial infarction, history of heart failure, history of cancer, atrial fibrillation, bundle branch block, serum creatinine, current admission due to ST-segment elevation acute myocardial infarction, peak troponin I, involvement of proximal left anterior descending artery, PCI, revascularization surgery, and complete revascularization. This analysis resulted in 2 groups with 555 patients paired according to whether or not they were receiving beta-blockers at discharge. The model used to generate the propensity score showed a 0.88 predictive capacity (95% confidence interval [95%CI], 0.87-0.89; P<.001), and a good fit (Hosmer-Lemeshow, P=.68). In the matched cohort, event-free survival was analyzed using the Kaplan-Meier method and the log rank test for between-group comparison.
We then built a multivariate Cox regression model, stratifying by the variable that identified each of the 555 patient pairs in the study. In this model, we included beta-blocker treatment as well as the other mortality-associated variables from the univariate Cox analysis. Discrimination was 0.82 in the multivariate Cox model. We checked the proportional hazards assumption with log-minus-log graphs and Martingale residuals. We calculated HR (hazard ratios) and their 95%CI for each variable and plotted the variables that showed significant association with mortality during follow-up.
Statistical analyses were performed with SPSS (Statistical Package for Social Sciences) version 18.0 for Windows. Statistical significance was defined as P<.05.
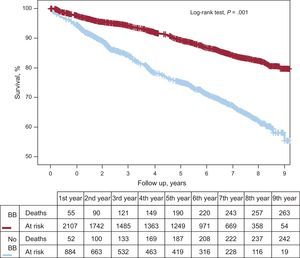
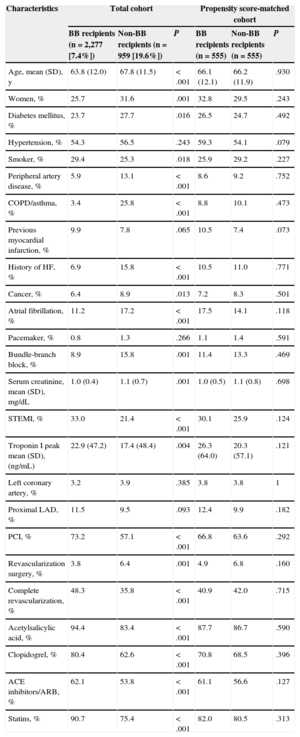
RESULTSBaseline Characteristics of the Total CohortThe initial cohort was comprised of 3236 patients with ACS and LVEF ≥ 50%. Mean age was 65.0 years (12.0 years), 27.5% were women, 24.9% had diabetes mellitus, 29.6% had ST-segment elevation acute myocardial infarction, 69.7% had undergone PCI, 44.6% had achieved complete revascularization, and 2277 patients (70.4%) were discharged with beta-blockers. Patients treated with beta-blockers had a lower cardiovascular risk profile (younger age and lower percentage of diabetes mellitus and peripheral artery disease). They had a lower rate of ACS without ST-segment elevation and a higher rate of complete revascularization (Table 1). This patient group was also prescribed a higher percentage of dual antiplatelet agents, angiotensin-converting enzyme inhibitors, and statins. Total mortality during follow-up was 15.6% (n=506), and this was significantly higher among non–beta-blocker recipients (Figure 2).
Baseline Characteristics of the Different Groups in the Beta-blocker Treatment Study
| Characteristics | Total cohort | Propensity score-matched cohort | ||||
|---|---|---|---|---|---|---|
| BB recipients (n=2,277 [7.4%]) | Non-BB recipients (n=959 [19.6%]) | P | BB recipients (n=555) | Non-BB recipients (n=555) | P | |
| Age, mean (SD), y | 63.8 (12.0) | 67.8 (11.5) | < .001 | 66.1 (12.1) | 66.2 (11.9) | .930 |
| Women, % | 25.7 | 31.6 | .001 | 32.8 | 29.5 | .243 |
| Diabetes mellitus, % | 23.7 | 27.7 | .016 | 26.5 | 24.7 | .492 |
| Hypertension, % | 54.3 | 56.5 | .243 | 59.3 | 54.1 | .079 |
| Smoker, % | 29.4 | 25.3 | .018 | 25.9 | 29.2 | .227 |
| Peripheral artery disease, % | 5.9 | 13.1 | < .001 | 8.6 | 9.2 | .752 |
| COPD/asthma, % | 3.4 | 25.8 | < .001 | 8.8 | 10.1 | .473 |
| Previous myocardial infarction, % | 9.9 | 7.8 | .065 | 10.5 | 7.4 | .073 |
| History of HF, % | 6.9 | 15.8 | < .001 | 10.5 | 11.0 | .771 |
| Cancer, % | 6.4 | 8.9 | .013 | 7.2 | 8.3 | .501 |
| Atrial fibrillation, % | 11.2 | 17.2 | < .001 | 17.5 | 14.1 | .118 |
| Pacemaker, % | 0.8 | 1.3 | .266 | 1.1 | 1.4 | .591 |
| Bundle-branch block, % | 8.9 | 15.8 | .001 | 11.4 | 13.3 | .469 |
| Serum creatinine, mean (SD), mg/dL | 1.0 (0.4) | 1.1 (0.7) | .001 | 1.0 (0.5) | 1.1 (0.8) | .698 |
| STEMI, % | 33.0 | 21.4 | < .001 | 30.1 | 25.9 | .124 |
| Troponin I peak mean (SD), (ng/mL) | 22.9 (47.2) | 17.4 (48.4) | .004 | 26.3 (64.0) | 20.3 (57.1) | .121 |
| Left coronary artery, % | 3.2 | 3.9 | .385 | 3.8 | 3.8 | 1 |
| Proximal LAD, % | 11.5 | 9.5 | .093 | 12.4 | 9.9 | .182 |
| PCI, % | 73.2 | 57.1 | < .001 | 66.8 | 63.6 | .292 |
| Revascularization surgery, % | 3.8 | 6.4 | .001 | 4.9 | 6.8 | .160 |
| Complete revascularization, % | 48.3 | 35.8 | < .001 | 40.9 | 42.0 | .715 |
| Acetylsalicylic acid, % | 94.4 | 83.4 | < .001 | 87.7 | 86.7 | .590 |
| Clopidogrel, % | 80.4 | 62.6 | < .001 | 70.8 | 68.5 | .396 |
| ACE inhibitors/ARB, % | 62.1 | 53.8 | < .001 | 61.1 | 56.6 | .127 |
| Statins, % | 90.7 | 75.4 | < .001 | 82.0 | 80.5 | .313 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, beta-blockers; COPD, chronic obstructive pulmonary disease; HF, heart failure; LAD, left descending artery; PCI, percutaneous coronary intervention; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as percentaje or mean (standard deviation).
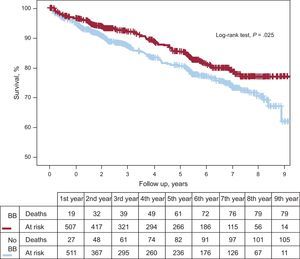
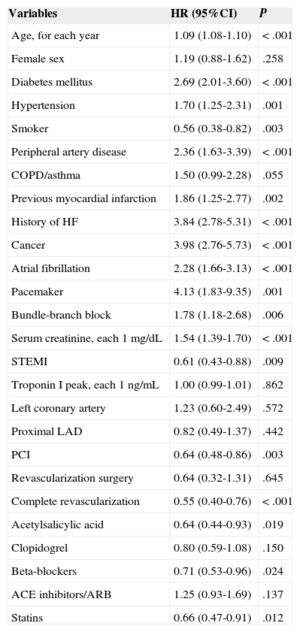
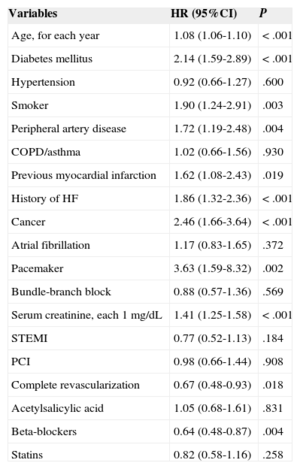
Propensity score matching resulted in 555 pairs of patients with and without beta-blocker treatment. Table 1 shows baseline characteristic-matching of patients in the pair-matched cohort. We found no differences in therapeutic strategy or in treatments prescribed at discharge. Table 2 shows the variables associated with lower mortality during follow-up in the pair-matched cohort. Mortality during follow-up was significantly lower in beta-blocker recipients than in nonrecipients: 14.4% (mean, 4.4 [2.7] years) compared with 18.9% (4.2 [2.8] years), respectively; HR=0.71; 95%CI, 0.53-0.96; P=.025 (Figure 3). After adjusting for clinical variables, beta-blocker treatment was still an independent predictor of lower mortality during follow-up (HR=0.63; 95%CI, 0.47-0.85; P=.003). The following variables were also independently associated with mortality: diabetes mellitus, smoking habit, peripheral artery disease, history of myocardial infarction, heart disease or cancer, pacemaker, serum creatinine, and partial revascularization (Table 3).
Long-term Mortality Predictors in the Patient Cohort After Propensity Score Matching
| Variables | HR (95%CI) | P |
|---|---|---|
| Age, for each year | 1.09 (1.08-1.10) | < .001 |
| Female sex | 1.19 (0.88-1.62) | .258 |
| Diabetes mellitus | 2.69 (2.01-3.60) | < .001 |
| Hypertension | 1.70 (1.25-2.31) | .001 |
| Smoker | 0.56 (0.38-0.82) | .003 |
| Peripheral artery disease | 2.36 (1.63-3.39) | < .001 |
| COPD/asthma | 1.50 (0.99-2.28) | .055 |
| Previous myocardial infarction | 1.86 (1.25-2.77) | .002 |
| History of HF | 3.84 (2.78-5.31) | < .001 |
| Cancer | 3.98 (2.76-5.73) | < .001 |
| Atrial fibrillation | 2.28 (1.66-3.13) | < .001 |
| Pacemaker | 4.13 (1.83-9.35) | .001 |
| Bundle-branch block | 1.78 (1.18-2.68) | .006 |
| Serum creatinine, each 1 mg/dL | 1.54 (1.39-1.70) | < .001 |
| STEMI | 0.61 (0.43-0.88) | .009 |
| Troponin I peak, each 1 ng/mL | 1.00 (0.99-1.01) | .862 |
| Left coronary artery | 1.23 (0.60-2.49) | .572 |
| Proximal LAD | 0.82 (0.49-1.37) | .442 |
| PCI | 0.64 (0.48-0.86) | .003 |
| Revascularization surgery | 0.64 (0.32-1.31) | .645 |
| Complete revascularization | 0.55 (0.40-0.76) | < .001 |
| Acetylsalicylic acid | 0.64 (0.44-0.93) | .019 |
| Clopidogrel | 0.80 (0.59-1.08) | .150 |
| Beta-blockers | 0.71 (0.53-0.96) | .024 |
| ACE inhibitors/ARB | 1.25 (0.93-1.69) | .137 |
| Statins | 0.66 (0.47-0.91) | .012 |
95%CI, 95% confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; HF, heart failure; HR, hazard ratio; LAD, left descending artery; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation acute myocardial infarction.
Independent Predictors of Long-term Mortality in the Multivariate Cox Regression Analysis Performed in the Patient Cohort After Propensity Score Matching
| Variables | HR (95%CI) | P |
|---|---|---|
| Age, for each year | 1.08 (1.06-1.10) | < .001 |
| Diabetes mellitus | 2.14 (1.59-2.89) | < .001 |
| Hypertension | 0.92 (0.66-1.27) | .600 |
| Smoker | 1.90 (1.24-2.91) | .003 |
| Peripheral artery disease | 1.72 (1.19-2.48) | .004 |
| COPD/asthma | 1.02 (0.66-1.56) | .930 |
| Previous myocardial infarction | 1.62 (1.08-2.43) | .019 |
| History of HF | 1.86 (1.32-2.36) | < .001 |
| Cancer | 2.46 (1.66-3.64) | < .001 |
| Atrial fibrillation | 1.17 (0.83-1.65) | .372 |
| Pacemaker | 3.63 (1.59-8.32) | .002 |
| Bundle-branch block | 0.88 (0.57-1.36) | .569 |
| Serum creatinine, each 1 mg/dL | 1.41 (1.25-1.58) | < .001 |
| STEMI | 0.77 (0.52-1.13) | .184 |
| PCI | 0.98 (0.66-1.44) | .908 |
| Complete revascularization | 0.67 (0.48-0.93) | .018 |
| Acetylsalicylic acid | 1.05 (0.68-1.61) | .831 |
| Beta-blockers | 0.64 (0.48-0.87) | .004 |
| Statins | 0.82 (0.58-1.16) | .258 |
95%CI, 95% confidence interval; COPD, chronic obstructive pulmonary disease; HF, heart failure; HR, hazard ratio; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Our results corroborate the long-term benefit of beta-blockers to improve survival after ACS in patients with preserved left ventricular systolic function. Although beta-blocker treatment is generally recommended after ACS, evidence in patients with preserved LVEF is based on studies conducted before the advent of reperfusion and thrombolysis.5–13 Care should therefore be taken when extrapolating this evidence to the present day. In fact, one could hypothesize that beta-blockers may be less beneficial since the introduction of PCI, because ACS is now managed with dual antiplatelet agents, statins, and a wider use of invasive strategies.
To date, few studies have analyzed the protective role of beta-blockers in current registries. Such studies have only found benefit among high-risk patients,14 such as those with ventricular dysfunction,17 previous infarction,16 and multivessel coronary artery disease15. Therefore, there can be no clear recommendation regarding the current use of beta-blockers in patients with preserved LVEF after ACS who have undergone coronary revascularization.18
The evidence for systematic beta-blocker treatment after ACS as a key component in secondary prevention is based on studies conducted before antiplatelet therapy and PCI were introduced, such as the Cooperative Cardiovascular Project,5 Stockholm Metoprolol Trial,6 Goteborg Trial,7 Beta-Blocker Heart Attack Trial,8 Norwegian Metoprolol Trial,9 and APSI trial.13 All these trials showed lower rates of all-cause and cardiovascular mortality and of reinfarction over a 6-year period, although the biggest benefit was observed in the first year. Furthermore, several meta-analyses reinforced the protective role of beta-blocker treatment.10–12 However, all these clinical trials were conducted in patients receiving less effective therapy than the current standard of care, because dual antiplatelet treatment, angiotensin-converting enzyme inhibitors, and statins were not in full use, and percutaneous coronary revascularization was yet to be introduced. It is therefore unclear whether the benefits afforded by beta-blockers in earlier trials still apply today. The improved long-term survival gained from PCI together with new treatments could mask this benefit, especially in patients with preserved LVEF.18 In consequence, we need to reassess the efficacy of beta-blockers after ACS in the current era.
As far as we are aware, only 5 studies with this objective have been carried out to date, and only 1 of these focused on patients with preserved LVEF. The first study, published by Kernis et al15 in 2004, analyzed 2442 patients who underwent primary PCI. The authors showed that beta-blockers were independently associated with lower mortality and fewer major adverse cardiac events, and mainly benefited patients with reduced LVEF (< 50%) and with multivessel coronary disease. One year later, De Luca et al studied 1513 patients with ST-segment elevation acute myocardial infarction treated with primary angioplasty. They found that beta-blocker treatment at discharge was an independent protective factor for first-year mortality, but only in patients with anterior wall infarction.16 The third contemporary study, published in 2010, was based on data from the J-Cypher registry.17 The authors, Ozasa et al analyzed 910 patients who underwent primary PCI, and did not observe any association between beta-blocker use and long-term mortality in patients after ACS. However, in a subgroup of patients with LVEF<40%, a lower mortality rate was observed in the beta-blocker treatment group. A more recent study, conducted by Nakatani et al14 on behalf of the OACIS registry researchers, showed that beta-blocker treatment was not associated with a lower risk for mortality after ACS, although subgroup analyses did confirm this association in high-risk patients (defined by the GRACE [Global Registry of Acute Coronary Events] risk score) and those treated with diuretics. Only one published study has focused on patients with preserved LVEF. In 2014, Choo et al19 analyzed 3019 patients with acute myocardial infarction who underwent PCI and had LVEF ≥ 50% at discharge. The authors performed a multivariate analysis and showed that beta-blocker treatment was associated with a 36.7% reduction in 3-year mortality (95%CI, 13.7%-53.6%), which is similar to our study results (37% during a a follow-up > 4 years).
Advances in ACS treatment have made a substantial change to the natural history of the disease over the past 20 years. As a result, both mortality and morbidity have significantly decreased, and most patients are discharged with preserved systolic function after ACS. Beta-blockers reduce myocardial workload, and thus oxygen demand, via a reduction in heart rate and blood pressure.20,21 Diastolic prolongation can increase perfusion in an ischemic myocardium—particularly in the subendocardium—, limit infarct size, and reduce reinfarction risk.22,23 Similarly, it has been shown that early beta-blocker use in ACS reduces the incidence of supraventricular and malignant ventricular arrhythmias.24,25 For the above reasons, we know that beta-blockers are useful during the acute phase of ACS to reduce the chance of cardiac death, and also to mitigate angina symptoms.26 Beyond the acute phase, the benefit of beta-blockers is not so clearly established in the current era, but appears to be focused on patients with the highest cardiovascular risk. As mentioned above, of the 5 contemporary studies conducted in the PCI era that analyzed preserved LVEF as a patient subgroup, only 1 study showed that beta-blockers afford long-term benefit after ACS. Therefore, contrary to current clinical practice guidelines, it appears there is no clear consensus among cardiologists as to whether beta-blocker treatment is useful in patients with normal LVEF after ACS. This lack of data reveals the need to reassess recommendations for beta-blocker use and duration of therapy in this patient subgroup. This is particularly important because beta-blockers cause side effects, such as bradycardia, hypotension, bronchospasm, fatigue, decreased libido, depression, and new-onset diabetes mellitus.27
Our study corroborates the evidence that beta-blockers are beneficial after ACS, even in patients with preserved LVEF. In our study, this benefit was independent of age, ACS type, extent of multivessel disease, revascularization outcome, and adjuvant treatment. Beta-blocker treatment reduced mortality by a third, coinciding with the recent study published by Choo et al.19 We found that 70% of patients were discharged with beta-blocker treatment in our cohort of patients with an LVEF>50% after ACS, mean age > 65, over 25% of ACS type being ST-segment elevation acute myocardial infarction, and over 65% of patients receiving PCI. Patients treated with beta-blockers were younger and had a lower cardiovascular risk profile than those who did not receive beta-blockers. However, after propensity score matching to eliminate the risk differences between the beta-blocker recipient and non–beta-blocker recipient groups, and after adjusting for potential confounders associated with mortality during follow-up in the univariate analysis, we found that beta-blocker treatment at discharge was a predictor of lower mortality during long-term follow-up. These results coincide with the REACH registry28 findings in terms of the prognostic benefit of beta-blockers after the acute phase of ACS. In the REACH subgroup analysis, the authors showed that for patients with stable coronary artery disease but with a history of myocardial infarction, beta-blockers effectively reduce the composite outcome of cardiovascular death, nonfatal acute myocardial infarction, and nonfatal stroke. Therefore, current scientific evidence, albeit scarce and based on nonrandomized studies, tends to favor beta-blocker use after ACS in patients with preserved LVEF, independently of other factors. The benefit of beta-blockers in terms of reducing mortality has a major clinical impact, particularly in the current era, because the prognosis of ACS has improved as a result of optimized health care, coronary revascularization, and drug treatment, guided by careful risk stratification.29–31
LimitationsThis study has several limitations. First, it does not have a randomized design, and therefore is subject to the classical limitations and bias that are inherent to retrospective studies. Although propensity score analyses are more robust than traditional regression techniques, they have certain weaknesses compared to randomized clinical trials, such as not being able to correct certain unmeasured confounding factors. Second, we did not have access to data referring to the start of beta-blocker treatment during the hospital stay. Third, we did not have detailed information on beta-blocker treatment after discharge, such as type, daily dose, adherence, and discontinuation, nor did we know whether this treatment was started later, after discharge. These factors may influence the actual clinical impact of beta-blocker treatment.
CONCLUSIONSOur results suggest that in the current era of PCI, there is long-term prognostic benefit of beta-blocker treatment after ACS in patients with preserved left ventricular systolic function. This study provides new evidence that will strengthen future recommendations in clinical practice guidelines for managing patients with ACS after discharge.
CONFLICTS OF INTERESTNone declared.
We are grateful to all health care and other staff at the Servicio de Cardiología del Hospital Clínico Universitario de Santiago de Compostela, for their exceptional clinical work and for seeking excellence in health care.