Keywords
INTRODUCTION
In recent years, increasing evidence has become available supporting the role of inflammation in the development of atherosclerosis and the pathogenesis of coronary thrombosis.1-4 Recent studies have shown that increased levels of certain inflammatory markers in patients with acute coronary syndrome (ACS) are associated with an increased number of cardiovascular complications and a higher incidence of death, both in the short term and in the long term.5-10 However, the majority of these markers are not universally available, their cost is high, and results are not usually available immediately. Consequently, their usefulness is limited in day to day clinical practice.
Various publications have shown that increased white blood cell count (WBC) is associated with a higher incidence of cardiovascular disease and all-cause mortality in the general population.11-17 Recent studies have supported the prognostic value of the WBC as a predictor of the development of heart failure and death in both the short term and long term following ACS, particularly following acute myocardial infarction (AMI).18-27 However, less data is available in the literature concerning unselected populations in which the new definition of AMI is applied and long-term follow-up performed.28
The aim of this study was to assess the association between WBC at admission and long-term mortality in patients with non-ST-segment elevation AMI (non-STEMI) or with ST-segment elevation AMI (STEMI).
PATIENTS AND METHODS
Study Group and Protocol
A prospective study was performed in a population of 1118 consecutive patients admitted with a diagnosis of AMI between November 1, 2000, and February 28, 2003, in the Hospital Clínic i Universitari, Valencia, Spain. Patients were stratified according to changes recorded in the ST segment of the electrocardiogram on admission: 569 non-STEMI patients and 549 STEMI patients. Therapeutic regimens were established on the basis of the stratification. The inclusion criteria used were those of the American College of Cardiology and the European Society of Cardiology.29 The criteria for STEMI were as follows: an increase in the levels of markers of myocardial necrosis (troponin I>1 ng/mL); new ST elevation from the J point in 2 or more contiguous leads with an elevation of at least 0.2 mV in leads V1, V2, and V3, or at least 0.1 mV in the remaining leads during the first 24 hours following the onset of symptoms. Patients were also included if a new ST-segment elevation in the presenting electrocardiogram was associated with a recent episode of chest pain but in whom it was not possible to obtain analyses of myocardial necrosis markers due to premature death, or if obtained, did not have values indicative of myocardial necrosis.30 The criteria for definition of non-STEMI were as follows: increased levels of markers of myocardial necrosis (as for STEMI) along with the presence of either symptoms of ischemia or alterations of the ST segment (except persistent ST-segment elevation). The treatment strategy for each type of AMI was based on established national and international guidelines.29,31 The requirement for an invasive study and revascularization was left to the judgment of the attending cardiologist. It is noteworthy that none of these patients had been transferred from other hospitals due to poor clinical progress. Patients with infectious, systemic inflammatory, or hematologic disease at admission were excluded from the study.
Variables Included in the Study
The variables analyzed in both types of AMI were obtained at admission and within the following 24 hours.
The following variables were recorded: medical history including age, sex, arterial hypertension, smoking, dyslipidemia, diabetes mellitus, personal and family history of ischemic heart disease, and percutaneous and surgical revascularization; systolic arterial pressure and Killip class determined at admission; ST-segment deviation (>1 mm in at least 2 contiguous leads) and number of leads involved; serum creatinine (mg/dL); WBC (cells/mL). In addition, maximum troponin I concentration (ng/mL) was determined in non-STEMI patients, and in STEMI patients the additional variables of heart rate, new left bundle branch block, episodes of sustained ventricular tachycardia/ventricular fibrillation in the first 24 hours, site of the AMI, thrombolysis, and electrocardiographic indicators of reperfusion (reduction in ST-segment elevation of at least 50% 90 minutes after thrombolysis) were assessed.
Definition of Events and Follow-up
An event was defined as death by any cause during a maximum follow-up period of 2 years (median follow-up period in the study population of 10±2 months). Follow-up was undertaken in the outpatients clinic of our hospital or through telephone contact with a member of the medical staff.
Statistical Analysis
The WBC determined at admission was assigned to 1 of 3 categories (x103 cells/mL): WBC1<10, WBC2=10 to 14.9, WBC3≥15 The cutoff points were selected according to previous studies.18-20,25
Quantitative variables were expressed as means (SD) and comparisons were made between the 3 WBC categories by analysis of variance. Data that did not display a normal distribution were expressed as medians (interquartile range) and were compared using the Kruskal-Wallis test. Qualitative variables were expressed as percentages and compared using the χ² test. Cumulative mortality for each WBC category was presented using Kaplan-Meier curves and differences between the categories were assessed using the Peto-Peto-Prentice test. The Cox proportional hazard regression model was used for multivariate analysis. Multivariate models were constructed using systematically obtained data collected from all patients within the first 24 hours of admission, independently of the type of AMI. Variables described in the literature as having recognized prognostic value were included irrespective of their level of statistical significance; variables not described in the literature as having prognostic value were only included if P<.20 in the bivariate analysis. Once the initial models were established, they were simplified by step-down variable selection. The assumption of proportionality of the risk was evaluated via analysis of the Schoenfeld residuals and the functional form of the quantitative variables (log-linear relationship) was determined using fractional polynomials.32 The discriminatory power of the adjusted models was evaluated using Harrell's c index for censored data. The estimated coefficients were expressed as hazard ratios with the respective 95% confidence intervals. In all cases, P<.05 was considered statistically significant. Statistical analyses were performed using the STATA statistical software package version 8.2.
RESULTS
Baseline Characteristics of the Study Group
The WBC of the study population had a range of 3.1-35x103 cells/mL. The median of the population was 9.8x103 cells/mL with an interquartile range of 7.8-12.5x103 cells/mL. The baseline clinical and demographic characteristics were stratified separately for each type of AMI according to the previously established WBC categories (Tables 1 and 2).
Non-STEMI
The distribution of the non-STEMI population according to WBC category was as follows: 351 patients (62.9%) were in category WBC1, 176 (30.9%) in WBC2, and 35 (6.2%) in WBC3. The mean age of the patients was 70 (12.1) years and 65% were men. The proportion of patients with diabetes mellitus, Killip class >2, and troponin I levels >1 ng/mL displayed a monotonic increase from WBC1 to WBC3, while the percentage of men was inversely proportional to the WBC. No other significant differences were observed for other study variables (Table 1).
STEMI
The distribution of the STEMI population according to WBC category was as follows: 228 patients (41.5%) were in category WBC1, 239 (43.5%) in WBC2, and 82 (14.9%) in WBC3. The mean age of the patients was 65±13 years and 72.9% were men. In this type of AMI, the proportion of active smokers, Killip class >2 at admission, heart rate >100 beats per minute, systolic arterial pressure <100 mm Hg, an episode of sustained ventricular tachycardia/ventricular fibrillation in the first 24 hours, the number of leads with ST-segment elevation, and the appearance of new Q waves displayed a proportional increase from WBC1 to WBC3, while the relationship was inversely proportional for the proportion of patients above 65 years, those with a history of ischemic heart disease, and in patients who met electrocardiographic criteria for reperfusion (Table 2).
White Blood Cell Count and Overall Mortality
A total of 214 deaths (19.1% of the total population) were registered during follow-up: 105 (18.5%) in non-STEMI patients and 109 (19.9%) in STEMI patients.
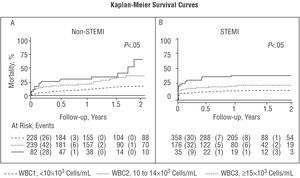
Bivariate analysis revealed that short-term (Tables 1 and 2) and long-term mortality increased proportionally between the WBC categories for both types of AMI (Tables 3 and 4). The Kaplan-Meier curves revealed separation of the groups according to WBC category from the earliest point in the follow-up, particularly in the STEMI patients (Figure 1B), and that these differences persist and even increase during follow-up for both types of AMI (Figure 1 A and B).
Figure 1. Significant differences in all-cause mortality according to white blood cell count category assessed using the Kaplan-Meier method for non-STEMI (A) and STEMI (B). WBC indicates white blood cell count; STEMI, ST-segment elevation myocardial infarction; non-STEMI, non-ST-segment elevation myocardial infarction.
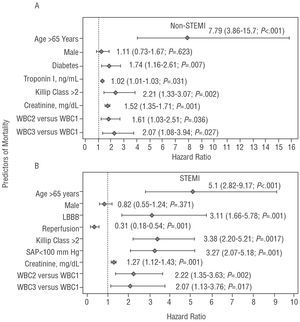
Figure 2. Predictors of all-cause mortality. Multivariate analysis for non-STEMI (A) and STEMI (B). WBC1 indicates white blood cell count <10x103 cells/mL; WBC2, white blood cell count of 10 to 14.9x103 cells/mL; WBC3, white blood cell count ≥15x103 cells/mL; STEMI, ST-segment elevation myocardial infarction; non-STEMI, non-ST-segment elevation myocardial infarction; LBBB, left bundle branch block; SAP, systolic arterial pressure.
Non-STEMI
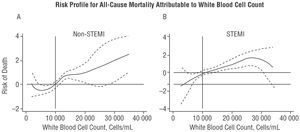
The final multivariate analysis of this group included only covariables obtained in the first 24 hours following the onset of symptoms (Figure 2A). The long-term risk of death compared with the WBC1 category was 1.61 (1.03-2.51; P=.036) and 2.07 (1.08-3.94; P=.027) times higher in categories WBC2 and WBC3, respectively (Figure 2A). Analysis of the functional form of the variable (fractional polynomials) showed that the risk of death attributable to WBC begins at 10x103 cells/mL and displays a linear increase from this point (Figure 3A). The c index for the multivariate model was 0.80 for this type of AMI.
Figure 3. Risk profile for long-term mortality attributable to white blood cell count in non-STEMI (A) and STEMI (B). Profile for non-STEMI adjusted for age, sex, diabetes mellitus, maximum concentration of troponin I, Killip class >2, and serum creatinine level. Profile for STEMI adjusted for age, sex, complicated left bundle branch block, electrocardiographic criteria for reperfusion, Killip class >2, systolic arterial pressure <100 mm Hg, and serum creatinine level. STEMI indicates ST-segment elevation myocardial infarction; non-STEMI, non-ST-segment elevation myocardial infarction.
STEMI
The final multivariate model for the whole group showed that the adjusted risk of death compared with category WBC1 was 2.22 (1.35-3.63; P=.002) and 2.07 (1.13-3.76; P=.017) times higher in categories WBC2 and WBC3, respectively (Figure 2B). Analysis of the functional form of the variable revealed that the risk of death attributable to WBC begins at just above 10x103 cells/mL; however, visual examination of the curve (Figure 3B) revealed a slight plateau beyond this point. The c index in this case was 0.85.
DISCUSSION
This study shows that WBC determined in the first few hours of AMI is a predictor of long-term mortality in the early risk stratification of STEMI and non-STEMI patients, independently of other variables of known prognostic value.
The literature contains an increasing amount of information that supports the prognostic value of inflammatory markers across a wide clinical spectrum of atherosclerotic disease, from their role in plaque pathogenesis to their usefulness in quantifying the inflammatory response during AMI.1-10
A number of epidemiological studies have shown that the baseline WBC is associated with an increased incidence of ischemic heart disease and mortality in the general population11-17 and there is current scientific interest in the potential prognostic value of the WBC determined during the acute phase of AMI to predict subsequent complications. Thus, recent studies have shown an association between an increased WBC and a higher incidence of complications following AMI, in particular, heart failure and short- and long-term mortality.18-28
A number of mechanisms have been proposed to explain this association: resistance to thrombolytic therapy due to alterations in the microcirculation,33 hypercoagulable state,34 a no-reflow phenomenon caused by leukocytes,35 indirect cardiotoxicity mediated by proinflammatory cytokines,36 promoters of ischemia-reperfusion injury,37 and expansion of the AMI. Regarding this final point, it is important to bear in mind that the leukocyte response that occurs following AMI is a central part of the inflammatory reparative respo nse that is initiated to replace the necrotic tissue with scar tissue. This may suggest that the greater the amount of necrosis, the larger the leukocyte response, an assertion based on experimental studies that show a direct relationship between the extent of necrosis and the level of both the local and the systemic leukocyte response.38,39 In addition, depletion of neutrophils in animal models in which coronary occlusion is performed leads to a significant reduction in the size of the infarct and the extent of reperfusion injury.40,41 In clinical settings, the extent of AMI is estimated using indirect parameters. Thus, various studies have related the WBC to variables associated with the size of the AMI: the development of heart failure,18,20,23,26,27 significant correlations with the peak level of isoenzyme MB of creatine kinase (CK-MB),18-19 or with left ventricular ejection fraction.21 In our sample, the proportion of the population with a Killip class >2 or a systolic arterial pressure <100 mm Hg showed a monotonic increase from one WBC category to the next in patients with either type of AMI. This was particularly apparent in the STEMI group, an observation that indirectly supports the association between WBC and the extent of AMI. Analysis of the functional form of the WBC within each multivariate model highlighted the following points: a) the WBC at which the risk begins to increase is around 10x103 cells/mL in both types of AMI, suggesting that this point can be considered as a possible cutoff for categorization; b) the slope of the risk curve in STEMI is shallower than in non-STEMI (Figure 3). The latter point may be explained by the greater prognostic impact of hemodynamic variables (Killip class and systolic arterial pressure) in STEMI than non-STEMI, as shown in the multivariate analysis for each type of AMI (Tables 3 and 4). This suggests the presence of a certain degree of colinearity between the WBC and variables related to the extent of the infarct, particularly in STEMI. The association is weaker in non-STEMI, suggesting that the WBC in these patients could be associated with baseline WBC, and as such, could be a valid indicator of the degree of systemic inflammation.
In our sample, despite adjusting for covariables associated with the size of the AMI, WBC acted as an independent predictor of long-term mortality. This finding provides indirect evidence in favor of an independent role for WBC in the pathogenesis of post-AMI complications.
We suggest that WBC is a useful biochemical tool for risk stratification of patients with either type of AMI. In particular, we would like to draw attention to the following logistic points:
1. Determination of WBC is systematically applied in clinical protocols for AMI and current clinical practice guidelines recommend basic blood analysis in response to chest pain consistent with coronary heart disease.
2. Analysis of WBC is widely available.
3. The WBC is obtained early: determination of the WBC in patients with AMI can be performed in the first few hours in any emergency department, unlike analysis of other inflammatory markers, which require reagents that are not normally available in an emergency department laboratory.
4. The cost of determining the WBC is low and, given that it is determined systematically, does not represent an additional cost in current procedures.
Limitations
The following represent inherent limitations in the study design: a) those limitations that are applicable to any observational study due to the difficulty of including variables with unknown prognostic value or that were not collected in our study; b) in the absence of a differential WBC at admission, we were unable to determine whether the prognostic value of the WBC was due to a specific component (e.g., neutrophils); c) the inclusion of only variables that can be collected during the first 24 hours following hospital admission prevented adjustment for other variables with known prognostic value that are usually assessed over the course of hospital stay, such as left ventricular ejection fraction.
CONCLUSIONS
The WBC determined at hospital admission in STEMI and non-STEMI patients was associated with long-term mortality, independently of other variables with known prognostic value. Consequently, we consider WBC to be a useful and widely available biological tool with which to identify patients at increased risk of death.
This work was supported by a grant from the Cardiovascular Research Network (RECAVA) of the Fondo de Investigación Sanitaria, Spain.
Correspondence: Dr. J. Núñez Villota.
Servei de Cardiologia. Hospital Clínic Universitari.
Avda. Blasco Ibáñez, 17. 46010 Valencia. España.
E-mail: yulnunez@gmail.com