The prevalence of hypertension has increased substantially in the last decades, with an estimate of 874 million people world-wide with systolic blood pressure values (SBP) of >140mmHg in 2015.1 Lowering blood pressure (BP) in hypertensive patients significantly reduces morbidity and mortality especially in persons with a history of cardiovascular disease.1 Despite numerous evidence underlining the importance of BP lowering and the widespread availability of effective and well-tolerated antihypertensive drugs, BP control rates remain unacceptably low. This is partly related to poor adherence to lifelong antihypertensive therapy but also, in a minority of patients, to resistant hypertension, defined as uncontrolled BP despite the intake of 3 antihypertensive drug classes in appropriate doses.2 Catheter-based renal denervation (RDN) is a relatively novel device-based treatment, targeting the sympathetic renal activity in minimally invasive ways and represents a potentially alternative treatment option for hypertensive patients.3 The first results were obtained from the open label SYMPLICITY HTN-1 and SYMPLICITY HTN-2 trials, along with several case series and observational studies.4,5 The SYMPLICITY HTN-3 trial6 proved safety but was unable to show efficacy of RDN using a radio-frequency catheter when compared with sham treatment in patients with severe resistant hypertension on multiple medication. Post-hoc analyses however, revealed several important information for patient selection, the dynamics of adherence to antihypertensive medication, and the relevance of revising technology and technique to RDN.7 Against this background, several novel, sham-controlled studies have been conducted and are, in part, published. SPYRAL HTN-OFF MED,8 SPYRAL HTN-ON MED,9 as well as RADIANCE-HTN SOLO10 showed significant and consistent reductions in BP, both office and ambulatory, in patients with and without concomitant antihypertensive drugs.
While randomized, controlled clinical trials remain the gold standard for the assessment of safety and efficacy of any novel approach in ideal conditions with high internal validity, there is great value for well-defined registries collecting real-world data from multiple geographies.11 These registries are particularly relevant for detection of rare events and for collecting information on novel devices use in real-world patients. Registries are an irreplaceable source of clinical research data to support long-term safety and effectiveness claims and for assessment of the external validity. In a recent paper published in Revista Española de Cardiología, Rodriguez-Leor et al.12 present the Flex-Spyral Registry, a multicenter registry of patients with resistant hypertension treated with RDN between 2009 and 2018 in Spain. A total of 125 patients (41% women, mean age 56±13 years) were included. Patients’ office BP was higher than 140/90mmHg at the time of inclusion (baseline BP, 160/95±20/16mmHg) despite treatment with 3 or more antihypertensive drugs (mean of 4.9±1.2 antihypertensive drugs) including at least one diuretic. The RDN procedures were performed in centers with specialized units for the management for hypertension, where secondary causes of hypertension had been ruled out before inclusion. At 6 and 12 months’ follow-up, a significant decrease in office BP by 16.5/8.0±24.2/15.8mmHg and18.7/7.0±25.1/14.8mmHg, respectively, was reported. Ambulatory BP and pharmacological treatment were also significantly reduced. Overall, the response rate defined as a decrease of systolic BP by a minimum of 10mmHg or of the 24-hour systolic BP by a minimum of 5mmHg to the RDN was 80.6%. Of note, between 2009 and 2015, RDN was performed using the Symplicity Flex (Medtronic Inc., Ireland) monopolar device and from 2015 to 2018 the Symplicity Spyral (Medtronic Inc, Ireland) tetrapolar catheter was used. Although of interest, neither the number of radiofrequency ablations nor detailed periprocedural data are presented.
The paper adds to the body of evidence indicating that RDN, when used in selected patients, can safely and effectively lower BP. Long-term data from the Global SYMPLICITY Registry (GSR) representing the largest available cohort of hypertensive patients receiving RDN in a real-world setting demonstrated both the safety and efficacy of the procedure with significant and sustained office and ambulatory BP reductions out to 3 years.11 Patient characteristics in the Flex-Spyral Registry presented herein are comparable to those in GSR in terms of age, sex, and body mass index. However, the GSR population included less smokers (10% vs 30%), yet the overall rate of cardiovascular comorbidities was almost twice as high (48% vs 26%). At baseline, office SBP was 166±20mmHg and office DBP was 95±16mmHg in the Flex-Spyral Registry, which was in line with office blood pressure values in the GSR (166±25mmHg and 90±17, respectively). Interestingly, there were some differences in antihypertensive drug treatment compared with the GSR, the use of aldosterone antagonists was more than twice as high in the Flex-Spyral Registry when compared with GSR (51% vs 25%). Aldosterone antagonists were the only substance group in both registries that has increased (58% and 28% after 12 months) over time, while administration of all other medication classes numerically decreased throughout follow-up. The increase of aldosterone antagonists is supported by publications of clinical trial data and guidelines for the management of arterial hypertension, in which spironolactone has been suggested as a drug of choice in patients with severe uncontrolled hypertension and may indicate care of hypertension excellence centers involved in the management of patients.13
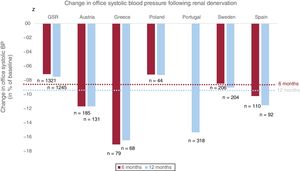
Once safety and efficacy have been proven by randomized clinical trials, novel therapies need to undergo further evaluation in usual conditions, as done by Rodríguez-Leor et al.12 for patients in Spain. This is particularly important for the assessment of long-term safety of RDN. These long-term analyses may be challenging in the setting of randomized clinical trials and therefore call for real-world data collection in registries, which should be monitored regularly for data accuracy and completeness. These analyses pave the way for the collection of cross-country comparisons over a longer period of time, representing all centers performing RDN as opposed to selected high-volume centers participating in the RDN pivotal trials. The collection of data in registries requires standardized protocols. One of its kind is the GSR representing the largest multicenter registry, with a prospective, single-arm design and follow-up to 3 years.11 Several other national registries are also enrolling patients. Figure 1 summarizes the overall changes in BP following RDN in all published European registries (Sweden,14 Austria,15 Portugal,16 Spain,12 Poland,17 and Greece18)–including the most recent results of the Spanish Flex-Spyral Registry published by Rodriguez-Leor et al.12
Change in office systolic blood pressure (BP) 6 months (red) and 12 months (blue) following renal denervation in large European registries and the Global SYMPLICITY Registry (GSR). Baseline office systolic BP: GSR, 166±25mmHg; Austria, 171±18mmHg; Greece, 176±15mmHg; Poland, 174±18mmHg; Portugal, 176±24mmHg; Sweden, 177±25mmHg; Spain, 166±20mmHg (for 6-months follow-up), 165±20mmHg (for 12-months follow-up). The red and blue dotted cross-line mark the average change in office systolic BP of all analyzed registries at 6 months (−8.3%) and 12 months (−9.6%), respectively.
In conclusion, registries are an important additional mean of data collection for treatments with proven short-term safety and efficacy such as RDN. Rodriquez-Leor et al. should be congratulated on completing this coherent national registry.12 Once the pivotal studies in RDN have been completed and the technologies have ultimately proven their efficacy and safety, they may be used in selected patients in clinical practice. It remains of utmost importance then, that all patients undergoing RDN are integrated in registries and one may speculate that data collection and completeness may be increased, when reimbursement is linked to registry participation. When feasible, these registries should be connected to national administrative health databases to automatically retrieve information about vital status, causes of hospitalization and causes of death, which may facilitate detection of long-term safety and efficacy.
FUNDINGF. Mahfoud is supported by Deutsche Forschungsgemeinschaft (SFB TRR 219), Deutsche Gesellschaft für Kardiologie (DGK).
CONFLICTS OF INTERESTF. Mahfoud has received scientific support and speaker honoraria from Medtronic and ReCor Medical. The remaining authors have no disclosures to report.
.